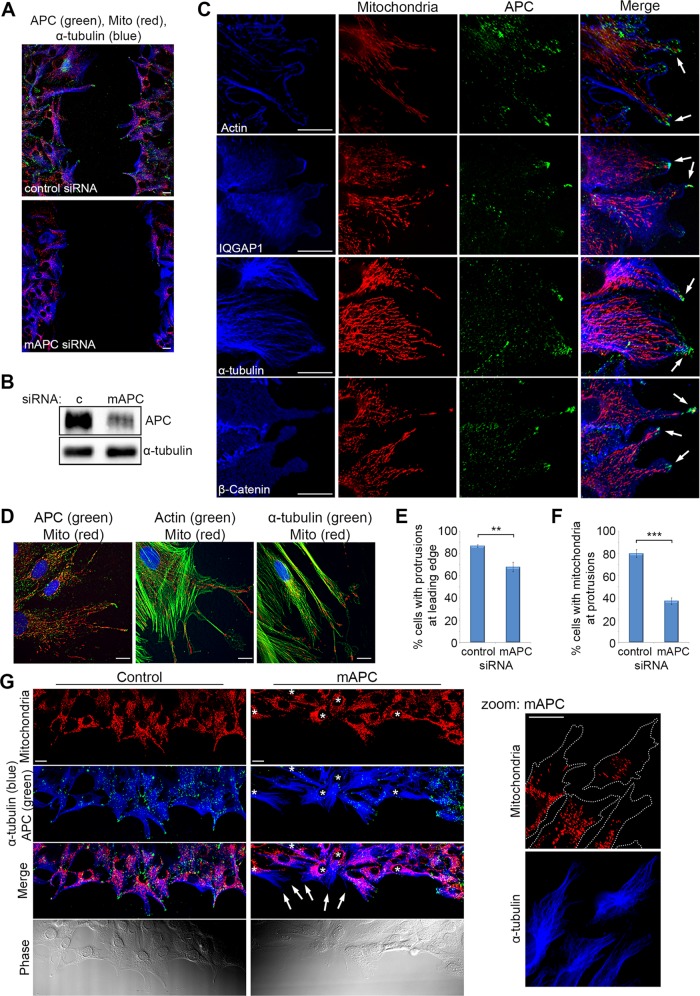
FIGURE 5:
Localization of mitochondria at microtubule-dependent cellular protrusions of actively migrating fibroblasts is lost upon APC silencing. Confluent cells were wounded; fixed 5 h later; counterstained for mitochondria (CMX-Ros), APC, and proteins involved in cell migration (actin, IQGAP1, α-tubulin, and β-catenin); and then analyzed by DeltaVision at the leading edge of the wound. (A) NIH 3T3 cells were treated with control or mAPC siRNA and counterstained for mitochondria (CMX-Ros), APC, and α-tubulin. Immunofluorescence analysis of cells at the leading edge of the wound revealed that cell migration was slowed upon loss of APC. (B) mAPC knockdown in NIH 3T3 cells was confirmed by Western blot. (C) Analysis of untreated NIH 3T3 cells at the wound edge indicated colocalization between mitochondria, APC, and cell migration factors at the membrane (arrows). (D) Colocalization of mitochondria and cell migration factors was confirmed by microscopy in human HDF1314 fibroblasts. (E) Formation of microtubule-dependent membrane protrusions was scored to reveal that treatment with mAPC siRNA reduced the number of protrusions detected relative to control cells (**, p < 0.01). (F) At the remaining protrusions, loss of mAPC decreased mitochondrial localization at protrusions (***, p < 0.001). Bar graph data presented as mean (±SD), statistical analysis by unpaired two-tailed t test. (G) Representative images of cells at the wound edge after transfection of control or APC-specific siRNAs (reduced green staining indicated by *). Note that mitochondria locate less frequently at microtubule-dependent protrusions after APC knockdown (indicated by arrows, closer view in zoomed panel). Scale bars: 10 μm.