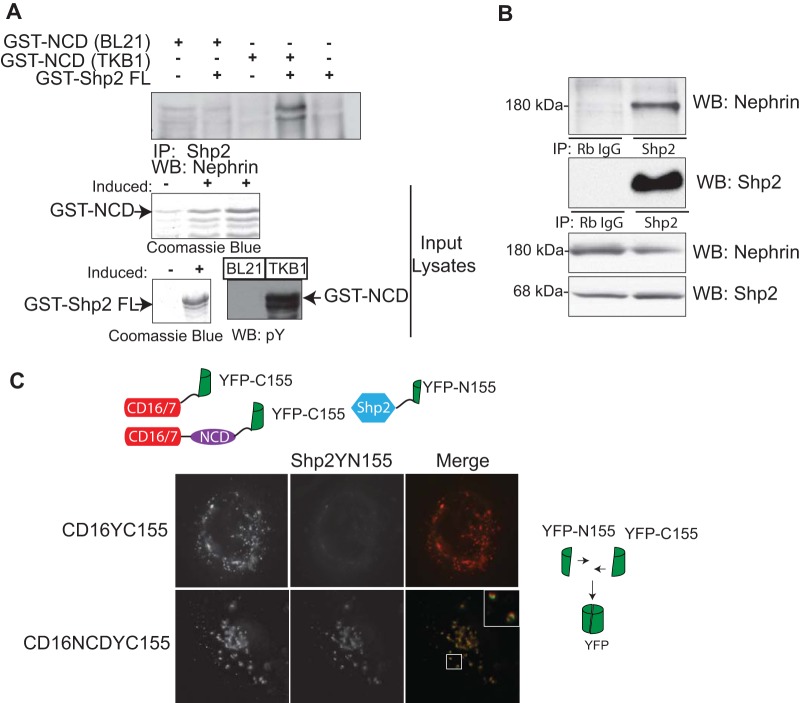
FIG 1.
(A) Shp2 binds to nephrin in a tyrosine phosphorylation-dependent manner. Purified recombinant full-length-GST-tagged Shp2 was incubated with tyrosine-phosphorylated GST-nephrin recombinant protein expressed in E. coli TKB1 or GST-nephrin expressed in E. coli BL21. Immunoprecipitation (IP) and immunoblotting (Western blotting [WB]) were performed with the indicated antibodies. Only tyrosine-phosphorylated nephrin associated with Shp2. Tyrosine phosphorylation of nephrin expressed in E. coli TKB1 was confirmed using phosphotyrosine antibody. Recombinant protein expression was confirmed using Coomassie blue. (B) Shp2 interacts with nephrin in glomerular lysates. A coimmunoprecipitation experiment using glomerular lysate showed interaction between endogenous nephrin and Shp2 (Rb IgG, rabbit IgG). (C) In vitro BiFC showing interaction of nephrin with Shp2. A construct encoding a chimeric protein with a CD16 extracellular domain, CD7 transmembrane domain, and nephrin cytoplasmic domain fused with a C-terminal fragment of YFP (YFP-C155) was generated. Full-length Shp2 was fused with an N-terminal fragment of YFP (YFP-N155). Following transfection in human podocytes, CD16 chimeras were clustered by the addition of anti-CD16 antibody (primary), followed by anti-mouse IgG (secondary) conjugated with rhodamine. Close approximation of the two YFP fragments resulted in reconstitution of the fluorescent protein and emission of green fluorescence. A CD16 construct fused with YFP-C155 lacking the nephrin cytoplasmic domain served as a control. Original magnification, ×630.