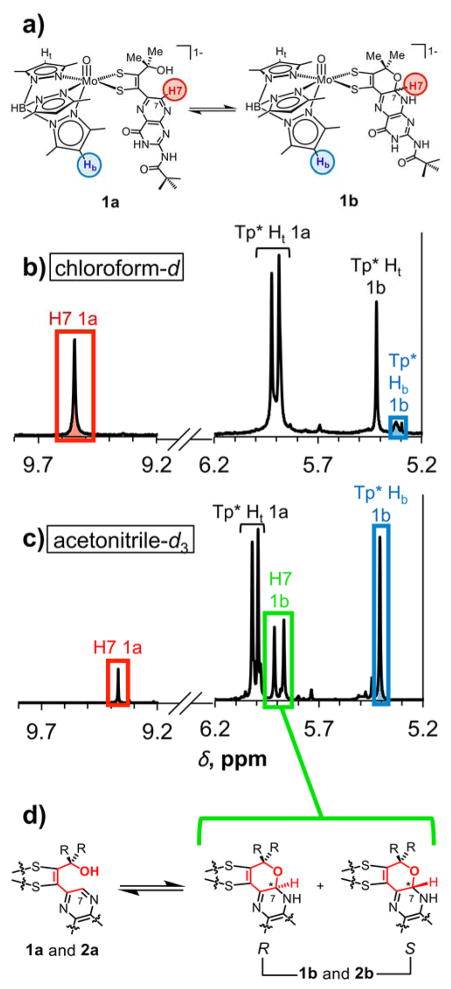
Figure 4.
(a) Structures of 1a and 1b highlighting corresponding protons used for integration data from 1H NMR spectra in (b) chloroform-d and (c) acetonitrile-d3; (d) equilibrium between the open form 1a or 2a and the two R and S diastereomers of the pyran forms 1b and 2b; two signals in the 1H NMR spectrum (highlighted in green in acetonitrile-d3) are for H7 in the pyran form, each corresponding to either the R or S isomer.