Abstract
Tissues develop in confined volumes that can impose mechanical constraints on their growth, but it is unclear how cells respond to these limits to regulate tissue size and shape. Two papers show that overcrowding and cell deformation lead to the shedding of live cells to maintain homeostasis in epithelial cell sheets.
How tissues acquire and maintain their size and shape is one of the mysteries of developmental biology. In nature, tissues do not develop in isolation, but in the presence of mechanical constraints imposed by their surroundings. Theoretical studies suggest that compressive forces could limit the rate of cell growth, producing a system in homeostasis in which cell proliferation and cell death are balanced (Shraiman, 2005). However, it is unclear how cells sense and respond to increases in cell number to prevent cell overcrowding that may compromise tissue function.
Overcrowding occurs naturally in epithelial tissues in vivo due to transient increases in cell division or migration. Though it has generally been assumed that cell death is the primary response of epithelial tissues to overcrowding, two recent studies show that excessive cells can be extruded without triggering cell death (Figure 1A) (Eisenhoffer et al., 2012; Marinari et al., 2012). Using an ingenious trick, overcrowding can be recapitulated and studied in culture. Epithelial cells grown to confluency on a stretched silicone membrane become overcrowded when that stretch is released, compressing the same number of cells into a reduced surface area (Eisenhoffer et al., 2012). The response to this sudden increase in cell density is surprising: a subset of nonapoptotic cells extrude from the epithelium until normal cell density levels are restored (Eisenhoffer et al., 2012). This cell culture assay makes it possible to follow the fates of the extruded cells and reveals that extruded cells are viable for several hours and can divide to confluency when harvested and regrown.
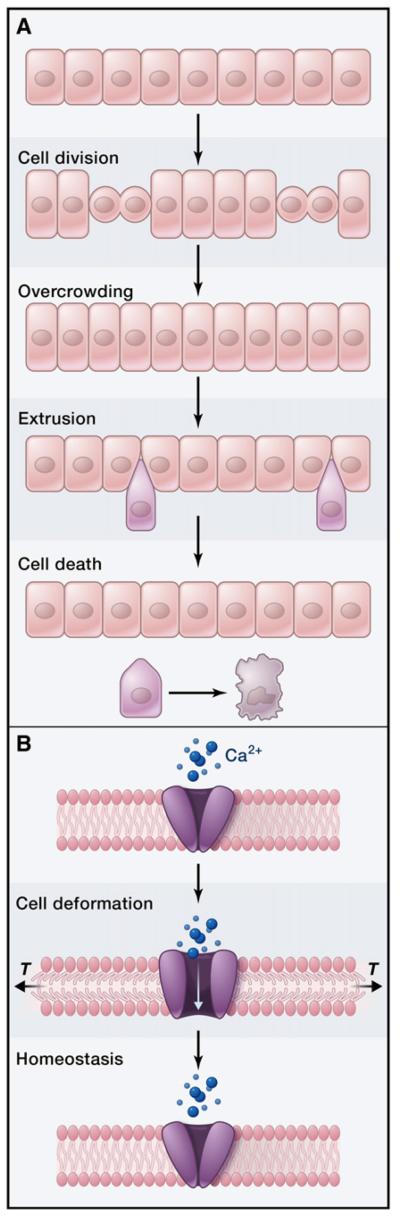
Figure 1. Live-Cell Extrusion Maintains Cell Density during Tissue Development and Homeostasis.
(A) Cell proliferation can generate overcrowding in an epithelium. Extrusion of cells in the overcrowded area relieves cell compression and returns the tissue to equilibrium. In cultured MDCK cells and the Drosophila notum, extruded cells are viable for a few hours before they undergo cell death, likely as a result of loss of adhesion to other cells or to the basement membrane.
(B) In one model, deformation of the cell membrane due to shape changes or overcrowding could open stretch-activated channels that promote extrusion through an unknown mechanism. Increased calcium influx, for example, could stimulate myosin light-chain kinase, activating myosin and promoting the assembly of a contractile ring around extruding cells.
Extrusion of living cells is also observed in vivo in the Drosophila notum, where nearly one-third of cells at the midline delaminate, compared to less than 1% of cells outside of the midline (Marinari et al., 2012). Delamination is not prevented by blocking apoptosis, indicating that the extruded cells are alive (Marinari et al., 2012). Delamination can be modulated by changes in cell density, as mutations that enhance cell growth increase delamination, and overexpression of tumor suppressors that inhibit cell growth results in fewer delamination events (Marinari et al., 2012). Regions with more delaminating cells are under less tension than their surroundings, raising the possibility that mechanical differences could signal cells to delaminate, although a causal relationship has not been determined. Consistent with this idea, cells that tend to extrude display increased cell shape anisotropy: midline cells are stretched parallel to the midline, their nuclei are displaced basally, and induction of overcrowding results in increased cell height (Marinari et al., 2012). These cell shape changes may generate three-dimensional mechanical asymmetries that could signal the need for extrusion. These studies provide experimental support for theoretical models proposing that mechanical forces can monitor cell growth and maintain homeostasis by triggering cell extrusion (Farhadifar et al., 2007; Marinari et al., 2012).
Like dying cells (Rosenblatt et al., 2001), living cells are extruded in response to crowding through the contraction of an actomyosin ring in the surrounding cells that helps to eject the cell from the epithelium (Eisenhoffer et al., 2012; Marinari et al., 2012). In support of this idea, laser ablation experiments show that the integrity of the ring is essential for extrusion (Marinari et al., 2012). Unlike dying cells exposed to UV light, live-cell extrusion in culture requires the activity of stretch-activated channels (Figure 1B), raising the possibility that mechanical cues could trigger a biochemical signal that promotes Rho-mediated actomyosin ring assembly and extrusion (Eisenhoffer et al., 2012). Pharmacological inhibition of stretch-activated channels blocks the extrusion of live, but not UV-irradiated, cells in culture, and using a photocleavable morpholino to target the Piezo1 stretch-activated channel in zebrafish causes cells to accumulate at the fin margins, resulting in large masses that resemble tumors (Eisenhoffer et al., 2012). Thus, the extrusion of living cells is a mechanism that can restore epithelia to normal cell density levels, presumably restoring normal tissue function.
These studies raise the question of how cell extrusion can be achieved without disrupting tissue integrity. Whereas cells that undergo apoptosis appear to shrink in size until they are eliminated from the sheet, extruding cells in the fly notum maintain cell adhesion and disassemble their contacts with their neighbors sequentially, reminiscent of neighbor exchange (Marinari et al., 2012). In fact, the authors propose that this form of delamination could restore hexagonal order to the tissue by removing irregularly shaped cells, although further studies will be necessary to test this idea. In the mouse intestine, tissue continuity following enterocyte extrusion is maintained by a basolateral redistribution of cytoskeletal regulators, tight junctions, and adherens junctions contacting the extruded cell (Guan et al., 2011). In developing epithelia, Rho-kinase and Abl kinase can phosphorylate Par-3 and β-catenin, respectively, displacing them from the cortex and priming adherens junctions for disassembly (Simões et al., 2010; Tamada et al., 2012). A similar mechanism may occur during cell extrusion in culture, which requires Rho-kinase activity (Rosenblatt et al., 2001; Eisenhoffer et al., 2012).
Cell extrusion induced by overcrowding could play a role in other processes in which cells are shed or internalized. Epidermal injuries cause the wound margins to retract, compressing cells outside of the wound. This transient crowding could provide a signal that activates the rapid extrusion of wounded cells. In the Drosophila embryo, furrows form during development in regions of increased cell density (Blankenship and Wieschaus, 2001) and cell shape anisotropy (Martin et al., 2010), raising the possibility that extrusion-like processes may be involved here as well. Finally, cell extrusion in epithelial tissues must be exquisitely regulated: infrequent extrusion could result in the formation of abnormal cell masses or tumors, and excessive extrusion could lead to invasive behaviors and metastasis. Investigating the mechanisms by which tissues sense mechanical constraints and activate cell extrusion during overcrowding and homeostasis could identify new avenues for cancer therapies designed to prevent tumor progression to metastasis.
REFERENCES
- Blankenship JT, Wieschaus E. Development. 2001;128:5129–5138. doi: 10.1242/dev.128.24.5129. [DOI] [PubMed] [Google Scholar]
- Eisenhoffer GT, Loftus PD, Yoshigi M, Otsuna H, Chien CB, Morcos PA, Rosenblatt J. Nature. 2012;484:546–549. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadifar R, Röper JC, Aigouy B, Eaton S, Jülicher F. Curr. Biol. 2007;17:2095–2104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Guan Y, Watson AJ, Marchiando AM, Bradford E, Shen L, Turner JR, Montrose MH. Am. J. Physiol. Cell Physiol. 2011;300:C1404–C1414. doi: 10.1152/ajpcell.00270.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinari E, Mehonic A, Curran S, Gale J, Duke T, Baum B. Nature. 2012;484:542–545. doi: 10.1038/nature10984. [DOI] [PubMed] [Google Scholar]
- Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. J. Cell Biol. 2010;188:735–749. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J, Raff MC, Cramer LP. Curr. Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- Shraiman BI. Proc. Natl. Acad. Sci. USA. 2005;102:3318–3323. doi: 10.1073/pnas.0404782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões Sde.M., Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, Zallen JA. Dev. Cell. 2010;19:377–388. doi: 10.1016/j.devcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada M, Farrell DL, Zallen JA. Dev. Cell. 2012;22:309–319. doi: 10.1016/j.devcel.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]