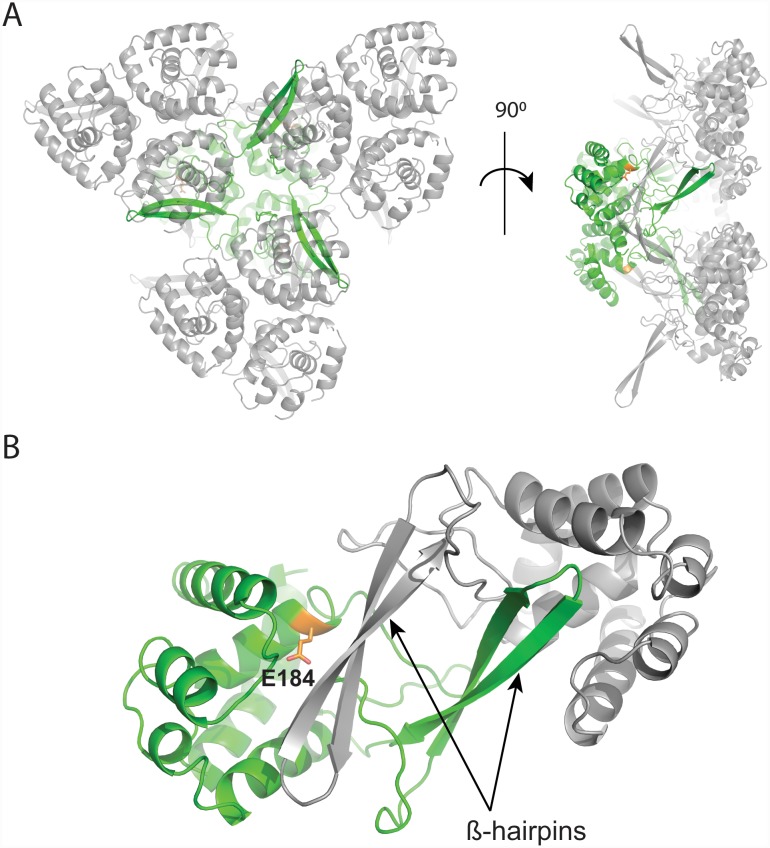
Fig 4. Crystal packing of StFlgJGH(151–301).
A) Packing of asymmetric units (ASUs) within the crystal structure of the C-terminally truncated mutant, StFlgJGH(151–301). Each ASU is comprised of three copies of StFlgJGH(151–301). One ASU is coloured green with three neighbouring ASUs coloured grey for clarity. B) The β-hairpin of each monomer of each ASU packs identically against the active site of a monomer within a neighbouring symmetry related ASU (grey) within the crystal structure. This packing imparts order to β-hairpin of each monomer while it partly occluding the active site of each copy of the protein in the crystal (conserved active site residue E184 shown in orange).