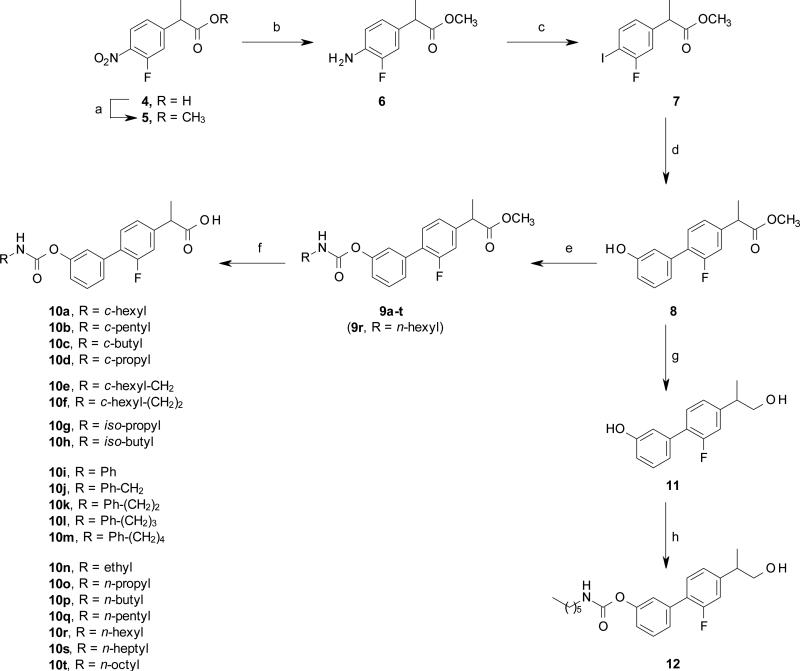
Scheme 1.
Synthesis of compounds 10a-t and 12. Reagents and conditions: (a) MeOH, conc. H2SO4, rt, 15 h, 93%; (b) HCO2NH4, 10% Pd/C, MeOH, rt, 3 h, 94%; (c) NaNO2, 3M HCl, 0 °C, 30 min, then NaI, 60 °C, 2 h, 55%; (d) (3-hydroxyphenyl)boronic acid, Pd(OAc)2, K2CO3, EGME/H2O, rt, 15 h, 84%; (e) RNCO, DMAP, MeCN, rt, 15 h, 38-99%; (f) 6M HCl, THF, rt, 2 d, 26-73%; (g) ZrCl4, NaBH4, THF, rt, 2 h, 96%; (h) n-hexyl-NCO, DMAP, MeCN, rt, 15 h, 73%.