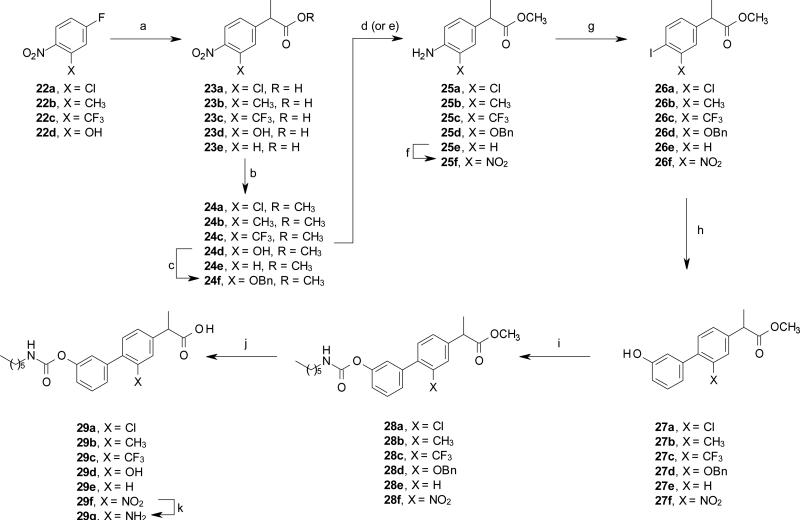
Scheme 6.
Synthesis of compounds 29a-g. Reagents and conditions: (a) diethyl methylmalonate, NaOH, DMF, rt, 15 h; then AcOH, H2SO4, H2O 110 °C, 24 h, 48-87%; (b) MeOH, conc. H2SO4, rt, overnight, 81-98%; (c) BnBr, K2CO3, acetone, 60 °C, 15 h, 63%; (d) Fe, HCl, MeOH, 65 °C, 2 h, 64-94%, for 24a and 24f; (e) HCO2NH4, 10% Pd/C, MeOH, rt, 3 h, quant., for 24b-c, and 24e; (f) Ac2O, HNO3 0 °C, 2 h then H2SO4, MeOH, 65 °C, 2 h, 98%; (g) NaNO2, 3M HCl, 0 °C, 30 min, then NaI, 60 °C, 2 h, 55-72%; (h) (3-hydroxyphenyl)boronic acid, Pd(OAc)2, K2CO3, EGME/H2O, rt, 15 h, 59-84%; (i) n-hexyl-NCO, DMAP, MeCN, rt, 15 h, 89%-quant.; (j) 2M HCl, dioxane, 80 °C, 15 h, 73-95%; (k) cyclohexene, 10% Pd/C 80 °C, 2 h then 2M HCl, 55%.