Table 1.
SAR exploration on the nature of R group: cycloalkanes, small-branched alkanes and phenyls.
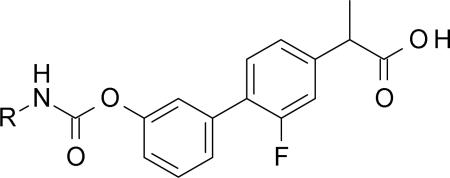
| ||||
|---|---|---|---|---|
| Compound | R | FAAHa,b IC50(μM)±SD | COX-1a,b IC50 (μM)±SD | COX-2a,b IC50 (μM)±SD |
| 2, URB597 | - | 0.0017±0.001 | >100 | >100 |
| 3a, flurbiprofen | - | >100 | 0.15±0.018 | 1.06±0.53 |
| 10a | c-hexyl | 8.2±2.4 | 7.9±2.1 | >100 |
| 10b | c-pentyl | 4.8±3.2 | 4.4±2.0 | > 100 |
| 10c | c-butyl | 48.7±9.0 | 0.72±0.02 | >100 |
| 10d | c-propyl | >100 | 5.4±2.9 | 74.3±6.1 |
| 10e | c-hexyl-CH2 | 0.36±0.06 | 0.60±0.04 | >100 |
| 10f | c-hexyl-(CH2)2 | 0.018±0.007 | 0.15±0.03 | 10.8±2.2 |
| 10g | iso-propyl | >100 | 3.9±2.1 | >100 |
| 10h | iso-butyl | 4.1±2.1 | 8.2±2.1 | >100 |
| 10i | Ph | 41.2±3.4 | 0.27±0.07 | 2.7±0.3 |
| 10j | Ph-CH2 | 4.18±2.8 | 1.3±0.6 | >100 |
| 10k | Ph-(CH2)2 | 0.17±0.07 | 6.3±2.2 | >100 |
| 10l | Ph-(CH2)3 | 0.09±0.01 | 0.58±0.09 | 6.2±0.3 |
| 10m | Ph-(CH2)4 | 0.027±0.010 | 3.7±2.8 | >100 |
Values are reported as mean values of ≥3 experiments performed
IC50 values were not determined for compounds showing less than 50% inhibition at concentrations of 100 μM for FAAH and COXs.
