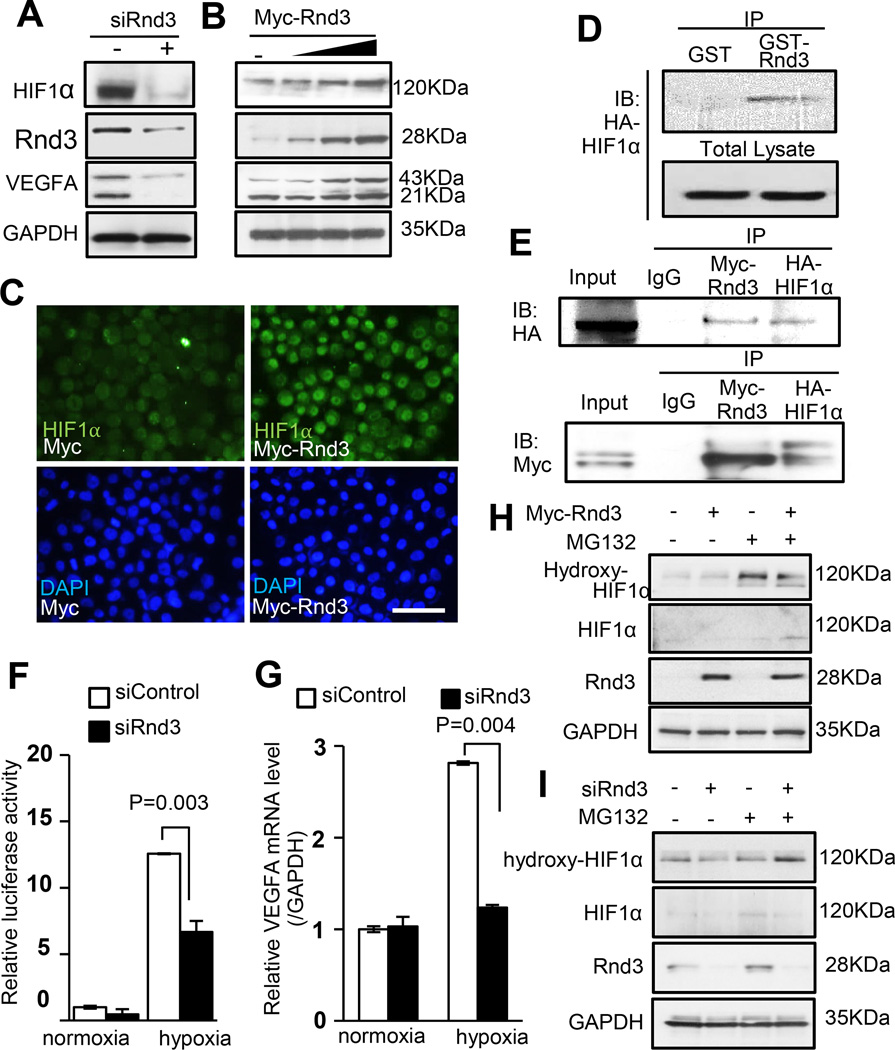
Figure 4. Rnd3 bound to HIF1α, prevented HIF1α protein from hydroxylation and stabilized the protein, which in turn promoted VEGFA expression.
(a) Downregulation of Rnd3 resulted in lower HIF1α and VEGFA protein expression. (b) The incremental increases in the expression levels of HIF1α and VEGFA protein were observed when Rnd3 was gradually overexpressed. (c) HIF1α nuclear accumulation was displayed when cells overexpressed Rnd3. Scale bar represents 50 µm. (d) HIF1α was pulled down by GST-Rnd3 protein in vitro. (e) Myc-Rnd3 physically interacted with HA-HIF1α in vivo evidenced by mutual coimmunoprecipitations. (f) Rnd3 deficiency weakened HIF1α transcriptional activity demonstrated by luciferase assays. (g) The qPCR analysis showed that VEGFA mRNA levels did not increase in the cells with Rnd3 deficiency in response to hypoxia. (h) Rnd3 overexpression in cells reduced hydroxylation of HIF1α. (i) Knockdown of Rnd3 led to an increase in Hydroxy-HIF1α levels. MG132: a proteasome inhibitor.