Abstract
Schistosomes, blood flukes, are an important global public health concern. Paired adult female schistosomes produce large numbers of eggs that are primarily responsible for the disease pathology and critical for dissemination. Consequently, understanding schistosome sexual maturation and egg production may open novel perspectives for intervening with these processes to prevent clinical symptoms and to interrupt the life-cycle of these blood-flukes. microRNAs (miRNAs) are key regulators of many biological processes including development, cell proliferation, metabolism, and signal transduction. Here, we report on the identification of Schistosoma japonicum miRNAs using small RNA deep sequencing in the key stages of male-female pairing, gametogenesis, and egg production. We identified 38 miRNAs, including 10 previously unknown miRNAs. Eighteen of the miRNAs were differentially expressed between male and female schistosomes and during different stages of sexual maturation. We identified 30 potential target genes for 16 of the S. japonicum miRNAs using antibody-based pull-down assays and bioinformatic analyses. We further validated some of these target genes using either in vitro luciferase assays or in vivo miRNA suppression experiments. Notably, suppression of the female enriched miRNAs bantam and miR-31 led to morphological alteration of ovaries in female schistosomes. These findings uncover key roles for specific miRNAs in schistosome sexual maturation and egg production.
Author Summary
Schistosomes are parasitic worms that cause the neglected tropical disease schistosomiasis. Schistosomes infect > 200 million people and lead to considerable morbidity, which is primarily due to egg deposition and the ensuing host immune response. Pairing with a male is a prerequisite for female sexual development and subsequent egg production. Thus, understanding schistosome development and egg production is important to unravel biological processes contributing to the life cycle and to understand the basic processes leading to the pathogenicity of schistosomiasis. Here, we show that schistosome miRNAs play an important regulatory role in schistosome sexual maturation and ovary development. Suppression of female enriched miRNAs bantam and miR-31 results in morphological alternation of ovaries in female schistosomes. Our findings reveal key roles for miRNAs in schistosome reproductive biology.
Introduction
Schistosomiasis is a human disease affecting over 200 million people worldwide and is caused by worms of the genus Schistosoma including S. haematobium, S. mansoni, and S. japonicum [1]. To date, no successful vaccine is available to prevent schistosomiasis [2]. The primary focus for control relies on chemotherapy using Praziquantel as the only widely applied drug [3,4]. This has raised serious concerns about the development of drug resistance, which would seriously compromise current treatment and control efforts [5]. This heavy reliance on a single drug and the risks it poses necessitates the identification of novel drug targets and/or the development of alternative strategies for schistosomiasis control.
Schistosomes are flatworms that are dioecious. Pairing of male and female worms is a prerequisite for female development and subsequent egg production [6–10]. The eggs are the major cause of pathogenesis of schistosomiasis and are essential for transmission of the disease [10–12]. Therefore, it is important to understand the molecular basis of schistosome sexual maturation and egg production.
Previous studies indicated that a continuous pairing contact is critical for female development [6–10,13]. Unmated female schistosomes are stunted in size and remain sexually immature. When paired female worms are separated from male worms, they cease egg laying and regress to an immature state. Re-introduction of males and their pairing with these immature females enables them to mature again [10,14–19]. Male-female pairing stimulates gamete development in females and leads to increased fertilization rates. Genomic [20], proteomic [10,12,21], and transcriptomic [21–25] studies have been used to interrogate the molecular basis of schistosome development and sexual maturation. Studies on male-female pairing [10,22,26–29] suggest that male schistosomes provide a key developmental signal that leads to female sexual maturation and egg production [13,15,30–37] ex reproduction.the gs. For example, transforming growth factor β (TGF-β) signaling is involved in the development of female vitelline cells and egg embryogenesis as a consequence of the direct interaction with male schistosome [38–42]. Furthermore, S. mansoni tyrosine kinases have been implicated in the regulation of schistosome gametogenesis [43]. Overall, these and other studies suggest that there are complex interactions within and between males and females that regulate female sexual maturation and egg production.
miRNAs, a class of small regulatory RNAs, are involved in the regulation of many biological processes primarily through the repression of messenger RNAs by typically binding to the 3’ untranslated region (3’UTR) of target mRNAs. miRNAs have been identified in several schistosome species including S. japonicum [44–47] and S. mansoni [48–51] and developmental stages including cercariae [45], lung-stage schistosomula [45], hepatic-stage schistosomula [44,45,52,53], adult males and females [50,54], and eggs [44]. Studies have identified miRNAs ranging in numbers from a few up to as many as two thousand [55,56]. However, only 79 mature S. japonicum miRNAs and 225 mature S. mansoni miRNAs are currently documented in miRBase (Version 21).
Several studies have described miRNAs that are differentially expressed between male and female schistosomes [45,50]. Marco et al found that 13 miRNAs are differentially expressed between males and females in S. mansoni [50]. miR-1b, miR-61 and miR-281 were highly expressed in males whereas miR-8447, miR-2f, mir-8437, miR-31, bantam, miR-2c, miR-2d, miR-71b, miR-36b and miR-755 were highly expressed in females. In S. japonicum, Cai et al demonstrated miR-7-5p, miR-61, miR-219-5p, miR-125a, miR-125b, miR-124-3p, and miR-1 were dominant in males, while bantam, miR-71b-5p, miR-3479-5p and miR-Novel-23-5p were predominantly found in the female parasites [45]. Considering the critical role that eggs play in the pathogenesis of schistosomiasis, miRNAs in schistosome eggs have also been analyzed and several miRNAs (sja-miR-71b-5p, sja-miR-71, sja-miR-1, sja-miR-36-3p, and sja-124-3p) were shown to be the most abundant in the egg stage [44]. In addition, the key molecules involved in miRNA biogenesis including Dicer [57–59], Argonaute proteins [57–60], and Drosha [58,59] have also been identified and shown to be differentially expressed in different stages and sexes of schistosomes. These studies suggest that miRNAs may act as important regulators in schistosome development, sexual maturation, and egg production. However, their functional and regulatory roles remain poorly characterized in schistosomes.
In this study, we used high-throughput small RNA sequencing to systemically identify S. japonicum miRNAs in the stages associated with male-female interaction, gametogenesis, and egg production. Using an Argonaute antibody-based pull-down assay and bioinformatic analyses, we identified the putative target genes for several of these miRNAs. Several of these targets were further validated by either in vitro luciferase assays or in vivo miRNA suppression experiments. In addition, suppression of female enriched miRNAs such as miR-31 and bantam led to morphological changes in the ovaries of female schistosomes. Overall, the results of our study indicate that miRNAs play a vital role in sexual maturation in S. japonicum. The development of a new strategy to target these miRNAs and thus reduce the egg production may help decrease parasite pathology and transmission in schistosomes.
Results
S. japonicum miRNAs
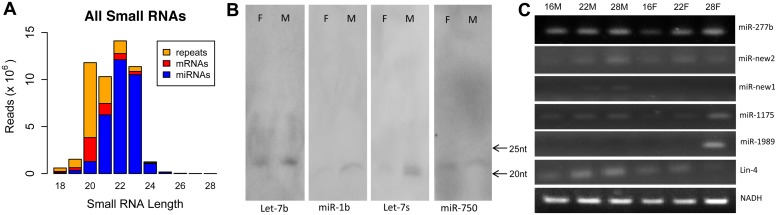
To identify S. japonicum miRNAs associated with male-female interaction, sexual development, and/or egg production, we isolated schistosomes from rabbits infected with S. japonicum cercariae at 16, 22, and 28 days post-infection, respectively. These stages represent the key stages of pairing initiation, gametogenesis, and egg production (S1 Fig). Paired males and females were separated, their RNA isolated, and small RNAs analyzed by deep sequencing. Eight small RNA libraries were prepared and analyzed including 16-day old females (16F), 16-day old males (16M), 22-day old females (22F), 22-day old males (22M), 28-day old females (28F), 28-day old males (28M), and mixed males (M) and mixed females (F). Bioinformatic analyses indicated that the majority of the small RNAs in these libraries were miRNAs and repeat associated siRNAs (S2 Fig). The majority of S. japonicum miRNAs are 21–23 nt in length, while repeat associated siRNAs are 20 nt in length (Fig 1A and S2B and S2C Fig).
Fig 1. Classification of S. japonicum small RNAs and analysis of newly identified S. japonicum miRNAs.
(A) Classification of S. japonicum small RNAs. Data represent the sum of small RNA reads from different stages and sexes for 8 S. japonicum libraries. Small RNAs that correspond to repetitive sequences (repeats, orange) and mRNA transcripts (red) are likely endo-siRNAs. (B) Northern blot analysis for S. japonicum miRNAs including let-7b, miR-1b, let-7s and miR-750. Total RNAs were isolated from 26-day old male and female S. japonicum. (C) Semi-quantitative RT-PCR analysis for the expression of the novel miRNAs in different stages and sexes of S. japonicum. 16M, 16-day old male; 22M, 22-day old male; 28M, 28-day old male; 16F, 16-day old female; 22F, 22-day old female; 28F, 28-day old female.
The library sequences were subject to de novo analyses for miRNA identification (S1 Table) [61]. Using relatively stringent criteria for defining miRNAs (see S1 File for criteria used to identify miRNAs), we identified 38 miRNAs from these stages (S2 Table). Of these, 10 miRNAs were new to S. japonicum (lin-4, miR-1b, let-7b, let-7s, miR-277b, miR-750, miR-1175, miR-1989, miR-new1 and miR-new2) and 6 are new to schistosomes (let-7b, let-7s, miR-750, miR-1989, miR-new2, miR-277b) (Table 1). We used Northern blots to independently demonstrate the expression of four (let-7b, miR-1b, let-7s, and miR-750) of these newly identified S. japonicum miRNAs (Fig 1B and S3 Table). The other six miRNAs (lin-4, miR-1989, miR-277b, miR-1175, miR-new1 and miR-new2) were below the level of detection of our Northern blots. However, the expression of these S. japonicum miRNAs was verified using an RT-PCR method (Fig 1C and S4 Table).
Table 1. Novel miRNAs identified in S. japonicum.
| Name | Sequences | Reads* | Hairpin locus |
|---|---|---|---|
| sja-let-7b | AGAGGUAGUGAUUCAUAUGACU | 655,165 | SJC_S000353: 80195–80367 |
| sja-let-7s | GAGGUAGUUAGAUGUACGACU | 111,993 | SJC_S000383:436660–436832 |
| sja-miR-750 | CCAGAUCUGUCGCUUCCAACU | 281,357 | SJC_S000460:180604–180776 |
| sja-miR-1175# | UGAGAUUCAAUUACUUCAACUG | 20,657 | SJC_S000460:180879–181001 |
| sja-miR-1989 | UCAGCUGUGUUCAUGUCUUCGA | 2,812 | SJC_S000128:160744–160623 |
| sja-miR-new1# | GAGAGAGCACUUUUAUGACGGA | 1,192 | SJC_S004484:20079–20188 |
| sja-miR-new2 | AGCUAAAUAGGUUAGUUUGACUGUC | 15,436 | SJC_S016196:787–876 |
| sja-lin-4# | UCCCUGAGACCUUAGAGUUGU | 23,558 | N/A |
| sja-miR-1b# | UGGAAUGUUGUGAAGUAUGUGC | 1,412,190 | N/A |
| sja-mir-277b | AAAAUGCAUCAUCUACCCUAGA | 207,027 | N/A |
* The number is the sum of miRNA reads in all of the 8 libraries.
# indicates that the miRNA has also been identified in S. mansoni.
Validation of miRNA expression in S. japonicum
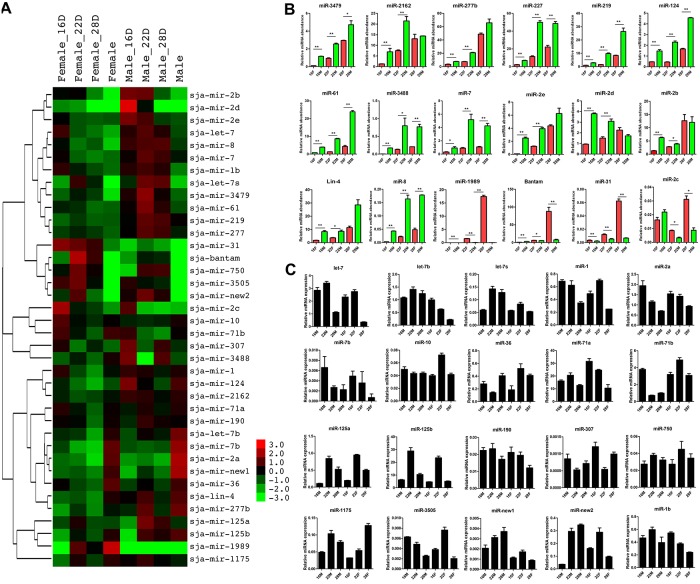
Analysis of miRNA expression using normalized reads (reads per million genome-matched reads [RPM]) demonstrated their differential expression in different stages of development and gender in S. japonicum (Fig 2A and S2 Table). Of the 38 S. japonicum miRNAs, 14 miRNAs were enriched in male worms whereas 4 miRNAs were predominantly found in females (Fig 2A and S2 Table). We further verified the differential expression of these sex-enriched miRNAs by qRT-PCR using independently prepared total RNA (Fig 2B). Our results demonstrate that these miRNAs were differentially expressed between male and female schistosomes (Fig 2B). We noted that the remaining 20 S. japonicum miRNAs were differentially expressed during schistosome development but showed no significant differential expression between males and females (Fig 2A). We used stem-loop based qRT-PCR to further validate the expression of these miRNAs during schistosome development (Fig 2C and S4 Table). The qRT-PCR data demonstrated these miRNAs were differentially expressed during schistosome development and that the expression data from qRT-PCR and deep sequencing in general correlated well (Fig 2C).
Fig 2. miRNA expression in S. japonicum.
(A) Hierarchical clustering of the miRNAs in mature females (F) and males (M) and during sexual maturation. The heatmap was constructed based on the log2 fold change values of normalized miRNAs across these stages, with 0 (black) being the average miRNA level (see S2 Table for the normalized expression values). (B) Sex-enriched miRNA expression was determined using qRT-PCR and total RNA isolated from 16F, 16M, 22F, 22M, 28F and 28M. For graphical representation, the ΔCt method was used to evaluate the relative expression of transcripts of miRNAs between males and females [62].* means P ≤ 0.05 and ** means P ≤ 0.01 (student’s t test, females vs males). Data illustrate representative findings and show the mean and standard errors derived from triplicate experiments. (C) Analyses of miRNA expression in different stages of S. japonicum. The expressions of 20 miRNAs that were differently expressed but do not show sex-biased expressions in S. japonicum were validated using stem-loop based qRT-PCR. Total RNAs were isolated from 16F, 16M, 22F, 22M, 28F and 28M. Data illustrate representative results with the mean and standard error derived from quadruplicate experiments.
Identification of miRNA targets
We predicted potential target mRNAs for these miRNAs using RNAhybrid software. To corroborate these predictions, we used antibodies against S. japonicum Argonaute proteins [60,63,64] (see Materials and Methods) to pull-down Argonaute miRNA/mRNAs complexes from paired male and female schistosome extracts and examined the accuracy of the mRNA target predictions (S3 Fig). RNA was isolated from Ago pull-downs, reverse transcribed, amplified by PCR, the PCR products cloned, recombinant clones randomly selected, and then sequenced (S3, S4A and S4B and S5 Figs and S5 Table) to determine if the predicted mRNA targets were enriched in the Argonaute pull-downs. The Argonaute enriched mRNA sequences were first used to identify the full-length mRNAs in NCBI database using BLAST, and then analyzed using RNAhybrid to predict miRNA:mRNA pairs. The overlapping mRNAs between the two methods were considered to be probable target mRNAs (Table 2 and S6 Table). Overall, this approach led to the corroboration of 30 potential target mRNAs for 16 of the described miRNAs.
Table 2. Validated target genes for S. japonicum miRNAs.
| miRNAs | Target gene IDs | Name of target genes | MiRNA:mRNA duplex* | Mfe (kcal/mol) | Validated # |
|---|---|---|---|---|---|
| Bantam | AY223092.1 | Serine-arginine repressor | Target 5`U CUUU A U 3` | -22.5 | By miRNA suppression |
| GGCU AUC CGAUCUCG | |||||
| UCGA UAG GCUAGAGU | |||||
| miRNA 3`AAU C 5` | |||||
| FN323394.1 | FUS-interacting serine-arginine-rich protein 1 | Target 5`G UU A U 3` | -22.6 | By luciferase assay/ miRNA suppression | |
| GGUUUU AUC CGAUCUCG | |||||
| UCGAAA UAG GCUAGAGU | |||||
| miRNA 3`U C 5` | |||||
| AY815078.1 | Smad1 | Target 5`U UG C 3` | -19.9 | By miRNA suppression | |
| GGC UUGAUCGUG CUUA | |||||
| UCG AAUUAGCGC GAGU | |||||
| miRNA 3`A UA 5` | |||||
| Let-7 | FN314191.1 | Ribosomal protein S6 kinase 2 | Target 5`A C U A 3` | -28.6 | By luciferase assay |
| CAU CAAC GAACUACCUC | |||||
| GUG GUUG CUUGAUGGAG | |||||
| miRNA 3`UG U G5` | |||||
| miR-2a | FN321618.1 | Plasminogen activator inhibitor 1 | Target 5`A AG CUGAAU C3` | -24.7 | By luciferase assay |
| GUUCA GA UUGGCUGUG | |||||
| CAAGU UU GACCGACAC | |||||
| miRNA 3`G AG AU U5` | |||||
| miR-31 | EU370927 | Frizz7 | Target 5`G G G 3` | -22.0 | By luciferase assay |
| UCGUCGUGGUU UUGU | |||||
| AGCGGCAUUAG AACG | |||||
| miRNA 3`UCGA GU5` | |||||
| FN319623.1 | O-glycosyltransferase | Target 5`U C 3` | -28 | By miRNA suppression | |
| GCUUU UUGUAAUCUUGCC | |||||
| CGAAG GGCAUUAGAACGG | |||||
| miRNA 3`U C U5` | |||||
| miR-1989 | FN317226 | Asparagine-rich protein | Target 5`A U AUA UA U 3` | -26.4 | By luciferase assay |
| C AAGA GUGA ACACAGUUGA | |||||
| G UUCU UACU UGUGUCGACU | |||||
| miRNA 3`A C G | |||||
| miR-8 | FN313640 | Integral membrane protein GPR177 | Target 5`U AC UUAUUU G3` | -22.3 | By luciferase assay |
| GCAUUUU UACCUA AUAGUA | |||||
| CGUAGAA AUGGAU UGUCAU | |||||
| miRNA 3`C AAU5` | |||||
| DQ643829.2 | Wnt | Target 5`C A 3` | -20.8 | By luciferase assay | |
| GGCAUC ACC GC GUAUUA | |||||
| CCGUAG UGG UG CAUAAU | |||||
| miRNA 3`AAA AU U 5` | |||||
| miR-3479 | FJ753578.1 | Transforming growth factor receptor II | Target 5`A U ACUUA U3` | -20.3 | By luciferase assay |
| CGA GC UAAGUGCAAUA | |||||
| GUU CG AUUCACGUUAU | |||||
| miRNA 3`C CUUCC 5` |
*miRNA:mRNA pair analysis was performed using RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
#luciferase assay = miRNA mimics down-regulate target mRNA sequences in mammalian cells; miRNA suppression = transfection of antisense miRNA sequences into schistosomes leads to increases in target mRNAs
Validation of miRNA targets
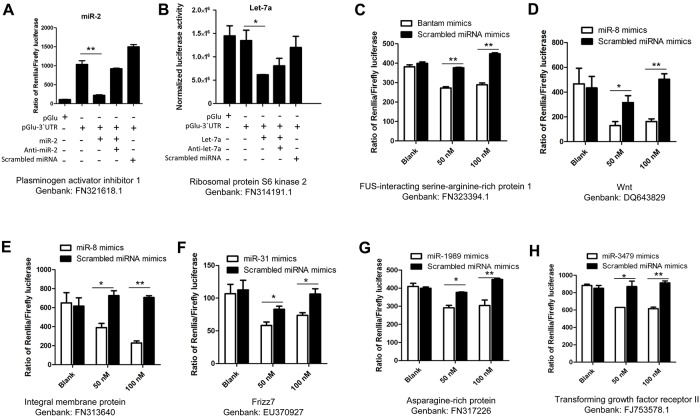
To validate the potential miRNA targets identified above, we determined whether miRNA mimics could repress luciferase mRNAs containing the target schistosome mRNA regions in mammalian cells. The target regions of potential miRNA binding sites for 9 predicted target mRNAs were selected and cloned into the 3’ UTR of a luciferase reporter vector (pGLU-CMV) (Fig 3 and S7 Table). Hela or HEK293T cells were transfected with these recombinant plasmids, control plasmids (pGL3), and the corresponding 2`-O-methyl and phosphorothioate miRNA mimics representing bantam, miR-8, miR-31, miR-1989, miR-3479, or control mimics (scrambled or mismatched seeding region of these miRNA) (S8 Table). As shown in Fig 3, transfection of bantam and miR-31 miRNA mimics resulted in a reduction of luciferase activity compared to transfection with scrambled miRNA mimics, indicating that these miRNAs can down-regulate the expression of the corresponding schistosome mRNA target regions in a heterologous system. Similar results were also observed in cells transfected with miR-8, miR-3479 and miR-1989 mimics and the corresponding recombinant plasmids expressing schistosome mRNA target sites (Fig 3). Furthermore, co-transfection in these experiments with antisense miR-mimics (miR-2 and let-7a) led to de-repression of luciferase (Fig 3A and 3B). We verified 8 target mRNAs for 7 miRNAs. Only one target mRNA for miR-277 failed to be validated in the luciferase assays. Overall, these data demonstrate the accuracy of our target mRNA predictions, indicate that schistosome miRNA sequences can effectively repress specific mRNA targets in mammalian cells, and that these miRNAs are likely to target these mRNAs in schistosomes.
Fig 3. Verification of predicted schistosome miRNA targets in mammalian cells.
Transfection of miR-2 (A), Let-7a (B), bantam (C), miR-8 (D and E), miR-31 (F), miR-1989 (G), and miR-3479 mimics (H) into HEK293T or Hela cells led to a significant reduction of luciferase activity from co-transfected plasmids containing their corresponding target regions. Hela or 293T cells were transfected with a recombinant Gaussia luciferase plasmid (pGLU-CMV) containing the corresponding S. japonicum cDNA fragment of a predicted mRNA targeted by a miRNA. These cells were simultaneously co-transfected with a control firefly luciferase plasmid. At 24 h post-transfection, a control miRNA or miRNA mimics were transfected into these cells. At 24–48 h post-transfection, luciferase activity was analyzed using a dual-luciferase reporter assay system with normalization to either firefly luciferase levels or protein concentration. For A and B, all the samples within the assay are illustrated, whereas in C-H only the effects of the miRNA mimics and scrambled miRNA mimics are shown. Similar results as illustrated in A and B were observed for the experiments in C-H. Blank represents no miRNA applied. Each experiment shows representative results and illustrates the mean and standard errors derived from triplicate experiments. * means P ≤ 0.05 and ** means P ≤ 0.01 (student’s t test, miRNA mimics treatment vs scrambled miRNA mimics treatment).
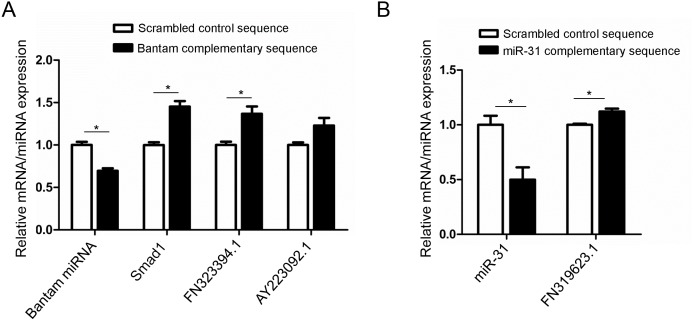
To determine whether these miRNAs could repress mRNA targets in vivo in schistosomes, we introduced bantam and miR-31 antisense miRNAs by electroporation into in vitro cultured adult female schistosomes and determined if their target mRNA levels changed using qRT-PCR. As shown in Fig 4A, in vivo suppression of bantam resulted in an increase in all three putative target mRNAs (GenBank accession numbers: AY815078, AY223092.1, and FN323394.1) including a target that was independently verified in our mammalian cell assays (S9 Table). Similar results were also observed in the schistosomes treated with antisense miR-31 (Fig 4B). Although another important evaluation for these in vivo assays would be to measure proteins translated from the target mRNAs, antibodies to these proteins are currently not available. Overall, we verified 11 mRNA targets for 7 of the miRNAs using either luciferase assay or in vivo miRNA suppression experiments (Table 2). Our in vitro luciferase assay in mammalian cells and in vivo schistosome data demonstrate that these schistosome miRNAs can regulate the expression of the predicted mRNA targets.
Fig 4. In vivo de-repression of S. japonicum target mRNAs with antisense miRNAs.
(A) Effect of bantam suppression on the expression of its putative target genes. (B) Effect of miR-31 suppression on the expression of its putative target gene. Adult schistosomes were collected from S. japonicum infected mice at 26–28 days post-infection. The female schistosomes were electroporated with anti-miRNAs or scrambled anti-miRNAs and their effects on the levels of endogenous miRNA and predicted target mRNAs determined by qRT-PCR at 4 days post-electroporation. Data illustrate representative results with the mean and standard error derived from triplicate experiments. * means P ≤ 0.05 and ** means P ≤ 0.01 (student’s t test, miRNA inhibitor treatment vs scrambled miRNA inhibitor treatment).
Effect of miRNA suppression on schistosome ovary architecture
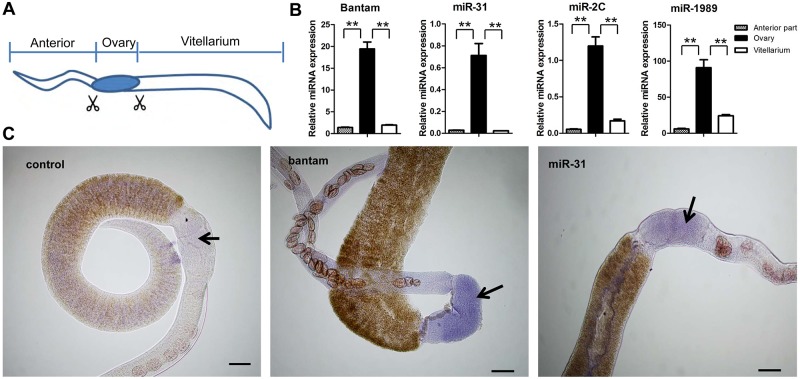
We reasoned that the female-enriched miRNAs might play an important role in S. japonicum sexual maturation and egg production. We dissected females into three parts (anterior, ovary, and vitellarium) and used qRT-PCR to examine the expression of the miRNAs in the ovary. Four female enriched miRNAs (miR-31, bantam, miR-1989 and miR-2c) were shown to be predominantly expressed in the region of ovary (Fig 5A and 5B). To further corroborate these results, we selected two of the female enriched miRNAs, bantam and miR-31, and used in situ hybridization to demonstrate that they predominantly localize to the ovary (Fig 5C and S10 Table). We next demonstrated that the target mRNAs of bantam (Smad1) or miR-31 (Frizz7) are also primarily localized in ovary of female schistosomes (S6 Fig and S10 Table). The co-localization of the miRNAs and their target mRNAs, at least for bantam miRNA and miR-31, suggest their coordinated involvement in the regulation of ovarian development.
Fig 5. Female enriched S. japonicum miRNAs are predominantly expressed in the ovary.
(A) Schematic diagram of the dissection of female schistosomes. (B) qRT-PCR analyses for the expression of four female enriched miRNAs (miR-31, bantam, miR-1989, miR-2c). * means P ≤ 0.05 and ** means P ≤ 0.01 (student’s t test, ovary vs. anterior and ovary vs. vitellarium). Data illustrate representative findings and show the mean and standard errors derived from triplicate experiments. (C) In situ hybridization experiments were carried out using a labeled locked nucleic acid (LNA) complementary to miR-31 or bantam miRNA. A labeled scrambled LNA was used as a control. Bars indicate 100 μm.
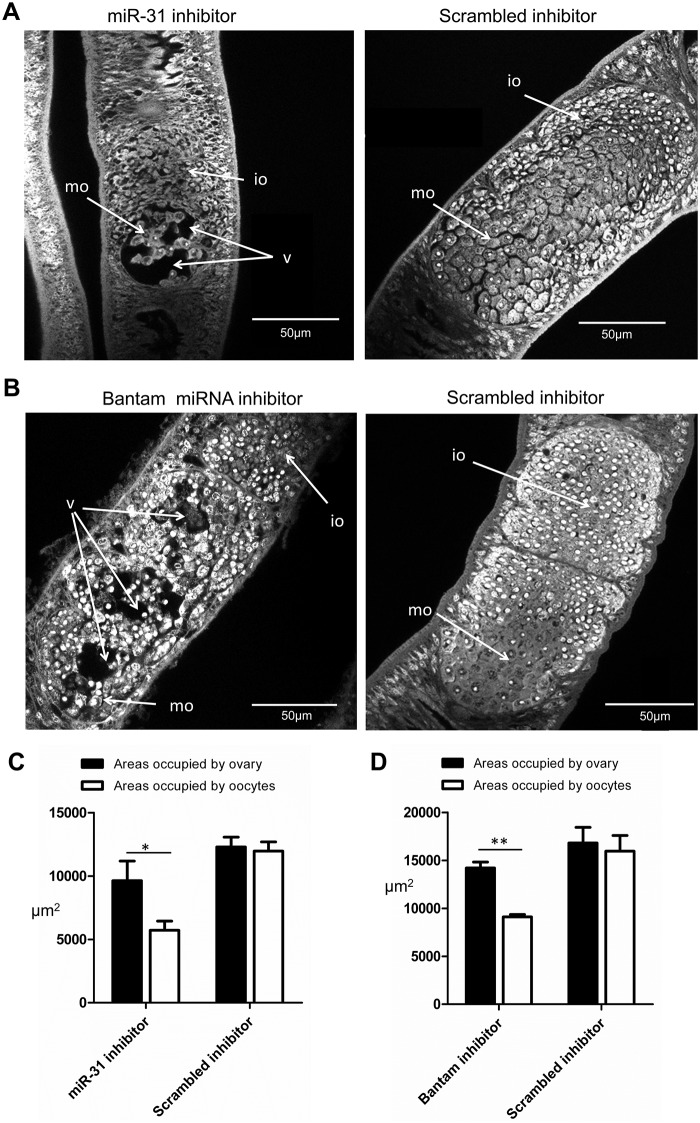
To test whether miR-31 and bantam miRNA suppression would lead to any morphological changes in female schistosomes, 24–28 day-old female worms were cultured in vitro for four days following electroporation of antisense miR-31 or bantam into worms. As controls, scrambled antisense miRNA were introduced into female worms. Upon transfection of 8–10 females with each miRNA inhibitor in a cuvette, we observed that at least 70% of the worms had significant defects in the ovarian architecture by confocal microscopy. Within the S. japonicum female ovary, immature oocytes mature into well-differentiated primary oocytes along an anterior-to-posterior gradient. We observed that there were gross-morphological defects for ovarian architecture in most of the treated females that included the formation of numerous vacuoles and the appearance of damaged oocytes (Figs 6A and 6B and S7–S10 and S1–S4 Movies). By examining the ratio of the total area of ovary to the area occupied by oocytes, miR-31/bantam inhibitor resulted in a significant reduction of parenchymal cells and oocytes in the ovary of female schistosomes (Fig 6C and 6D). Morphological changes in other female tissues were not observed, and no alterations were found within the ovaries of females treated with control antisense miRNA. These results suggest that bantam and miR-31 and their target mRNAs play important roles in the development of the structure and architecture of the ovary and in oocyte differentiation. We did not observe significant changes on other physiological parameters such as behavior, viability or mortality rates of target/antisense RNA-treated females compared with controls females treated with scrambled antisense miRNA (S11 Fig). Furthermore, RNA levels of miR-31 and bantam determined by qRT-PCR were also significantly reduced in target/antisense RNA-treated worms compared to control worms (S12 Fig).
Fig 6.
Effect of miR-31 (A) or bantam (B) suppression on female ovary architecture and morphology. Female schistosomes were electroporated either with miRNA inhibitors (antisense miRNA) (3 μg) or scrambled miRNA inhibitors (3 μg) and then incubated for 4 days as described in Materials and Methods. S. japonicum worms were stained with carmine red and whole-mount preparations of worms were imaged by confocal microscopy. Io, immature oocyte; mo, mature oocyte; v, vacuole. Quantitation of ovary changes due to miR-31 (C) and bantam miRNA suppression (D). The ovary defects were analyzed using ImageJ as described in Materials and Methods based on a comparison of the area occupied by the ovary and oocytes and the appearance of oocytes in the ovaries. Data show the mean and standard errors derived from four randomly selected female worms. * means P ≤ 0.05 and ** means P ≤ 0.01 (student’s t test, ovary areas vs areas of appearance of oocytes in ovary).
Discussion
A number of studies have investigated the molecular basis for schistosome egg production by determining and examining genes associated with reproductive development [8–10,12,13,21,65–71]. Although schistosome miRNAs have been identified in several different stages of S. mansoni [48–51,72] and S. japonicum [52–54] (including cercariae [45], lung-stage schistosomula [45], hepatic-stage schistosomula [45,46,52,53], adult males and females [54], and eggs [44]), the potential regulatory roles of small RNAs in schistosomes, and particularly in sexual maturation and egg production have not been previously investigated. Our study represents the first attempt to systemically characterize S. japonicum miRNAs associated with sexual maturation and egg production, to predict potential mRNA targets, to test these predictions in vitro and in vivo, and to determine whether repression of miRNAs has a consequence on the female reproductive system.
We identified 28 known miRNAs and 10 novel miRNAs associated with sexual maturation and egg production in S. japonicum. The 28 known miRNAs were previously reported to be expressed in other schistosome stages including eggs isolated from the host’s liver, cercariae and lung schistosomula [44,45], suggesting that these miRNAs likely play diverse roles in schistosomes. Among the 10 novel miRNAs, three of the miRNAs (let-7b, let-7s, and miR-750) have long hairpin structures (>200 nt), suggesting that schistosomes miRNA biogenesis has adapted to process these long hairpin precursor miRNAs. Three other novel miRNAs (lin-4, miR-1b and miR-277b) are significantly expressed (>20,000 reads in the libraries) and conserved in other metazoan although the current draft S. japonicum genome data lacks the predicted hairpin structures. Our Northern blot and Semi-quantitative RT-PCR results clearly demonstrated that the miRNA are expressed in S. japonicum.
Male-female pairing is essential for female sexual maturation. It has been suggested that the male schistosomes ensure physical transport, correct tissue localization, aid feeding, and provide maturation factors for female development and egg production [6–9,12,13]. We identified 14 male-enriched miRNAs and 4 female-enriched miRNAs using high-throughput sequencing, and their expression was independently verified by qRT-PCR. Several sex-enriched miRNAs were also previously identified as differentially expressed including sja-miR-7, sja-miR-61, and sja-miR-219 in male worms and sja-bantam in female worms [45,50]. We identified several target genes of miR-219 and bantam miRNA using an Argonaute antibody-based pull-down assay in combination with bioinformatic analyses. Furthermore, using antisense RNA to inhibit miRNAs, we demonstrated that bantam miRNA plays a regulatory role in ovary development and oocyte maturation. We further identified 30 potential target mRNAs for 16 S. japonicum miRNAs. Eleven of the targeted mRNAs for 7 of the miRNAs were further validated using an in vitro dual-luciferase reporter assay or in vivo miRNA suppression experiments (Figs 3 and 4 and Table 2). These data provide the first experimental demonstration of target prediction and verification both in vitro and in vivo in schistosomes.
Among the validated miRNA targets, we found that male enriched miR-8 and miR-3479 regulate the molecules likely involved in the Wnt (GenBank Accession Nos: FN313640 and DQ643829.2) and TGF-β (Accession No. FJ753578.1) signaling pathways. These pathways have been shown to be involved in the regulation of schistosome development [20,73] and embryogenesis [66,67,74]. Consequently, our results suggest that miRNAs may contribute to the regulation of Wnt and TGF-βpathways influencing the physiological processes associated with their activities [41,42].
We demonstrated that female-enriched miR-31 can regulate the mRNA for Frizz 7 (Accession No: EU370927, a receptor of WNT signaling pathway) and an O-glycosyltransferase (Accession No: FN319623.1) whereas the female-enriched miR-1989 likely interacts with asparagine-rich protein mRNA (Accession No. FN317226). As a member of the frizzled family of G protein-coupled receptors, Frizz 7 is a transmembrane receptor involved in multiple signal transduction pathways, particularly in modulating the activity of the Wnt proteins, which play a fundamental role in the early development of metazoa [75]. Asparagine-rich proteins are involved in the transcriptional regulation of key eukaryotic developmental processes [76] and O-glycosyltransferase is a catalytic enzyme in glucose metabolism involved in the transfer of activated carbohydrate moieties from donor molecules (e.g. UDP-galactose) to an acceptor molecule associated with the regulation of carbohydrate metabolism and/or other functions [77,78]. We also observed that bantam miRNA suppression leads to a significant increase in mRNA levels of three other identified targets, including a serine-arginine repressor (Accession No. AY223092.1), FUS-interacting serine-arginine-rich protein 1 (Accession No. FN323394.1), and Smad1. Serine-arginine repressor and FUS-interacting serine-arginine-rich protein 1 are members of the serine-arginine family of proteins involved in constitutive and regulated RNA splicing [79,80]. Moreover, studies in Drosophila [81] indicated that bantam was an effector of several signaling pathways such as Hippo [82,83], Notch [84], Dpp [85], and epidermal growth factor receptor (EGFR) [86] during fly development. We demonstrated in a previous study that S. japonicum bantam can be detected in the circulation of the definitive host [87], suggesting that bantam might be a secretory miRNA that has the potential to act as a signaling molecule for other schistosomes or even host cells [56]. Collectively, these results suggest that female enriched miRNAs may be involved in a variety of processes including signal transduction for schistosome sexual maturation and egg production.
miR-31 and bantam are predominantly present in the ovaries of female schistosomes (Fig 5), suggesting that these miRNAs may play an important role in ovary development. Suppression of these miRNAs led to significant defects in the ovaries. These findings strongly imply that female enriched miRNAs such as bantam and miR-31 may be key regulators in S. japonicum ovarian development. We also demonstrated that schistosome smad1 (a target gene of bantam) and Frizz7 (a target gene of miR-31) are predominantly localized in the ovary of S. japonicum, consistent with their localization in S. mansoni [88–90]. From these data, we conclude that bantam and miR-31 are involved in the regulation of target genes that are instrumental in ovary development and oocyte maturation. Overall, our data identify female enriched miRNAs and their mRNA targets that play important roles in sexual maturation in S. japonicum.
Taken together, our study represents the first attempt to systemically characterize S. japonicum miRNAs associated with sexual maturation and egg production by predicting miRNA targets and then using both in vitro and in vivo assays to functionally evaluate the predictions and examine the roles of the miRNAs in vivo. Importantly, our findings provide the first functional evidence for the role of miRNAs acting as important regulators in ovary development and sexual maturation in S. japonicum. Since schistosome eggs are the major cause of schistosome pathology and play a key role in the epidemiology of human infection, our studies have important implications for human schistosomiasis.
Materials and Methods
Ethics statement
All experiments involving mice and rabbits were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People's Republic of China, and all efforts were made to minimize suffering. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Permit Number: SHVRIAU-10-0101).
Animals and parasites
The life cycle of S. japonicum (Anhui isolate) was maintained in New Zealand rabbits and BALB/c mice using Oncomelania hupensis as the snail host. Unless indicated otherwise, New Zealand rabbits and BALB/c mice were infected with approximately 1,000 and 100 cercariae, respectively, by inoculating the shaved abdominal skin surface with a moist cercarial paste. To collect schistosomes at different developmental stages, the parasites were perfused from BALB/c mice every day starting at 14 days post infection through 35 days post-infection. Males and females were manually separated and pooled. Schistosomes at the developmental stages of pairing, gametogenesis, and egg production were collected from infected rabbits at 16, 22, or 28 days post-infection, respectively. Males and females were manually separated. The sexes of worms were confirmed by microscopic analysis. The parasites were snap-frozen and stored in liquid nitrogen until use for total RNA isolation.
RNA isolation
Total RNA was extracted from S. japonicum samples using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Samples included 16-day-old males (16M), 16-day-old females (16F), 22-day-old males (22M), 22-day-old females (22F), 28-day-old males (28M), 28-day-old females (28F), a male mixture (M) (pooled from 14–35 days), and a female mixture (F) (pooled from 14–35 days). To maximize precipitation of small RNAs, RNA precipitation with isopropanol was carried out overnight at -80°C. RNA was quantified using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE), and RNA quality was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies).
Small RNA libraries, sequencing, and bioinformatics
Small RNA libraries, sequencing, and bioinformatics are described in Supplemental materials and methods.
Northern blot and real time RT-PCR analyses of schistosome miRNAs
DNA oligonucleotides complementary to miRNA sequences were end-labeled with DIG (Invitrogen, Shanghai) at their 5’ termini and used as probes (S3 Table). Northern blot analyses were performed according to a previously described method [91] using 30 μg of total RNA isolated from adult male and female schistosomes isolated from BALB/c mice at 26 days post-infection.
For real time RT-PCR analyses of miRNA expression, schistosomes were collected from rabbits infected after 16, 22, and 28 days post-infection. Male and female worms were manually separated. Total schistosome RNA was isolated TRIzol (Invitrogen) from different developmental stages, sexes, and regions of female worms. Real-time RT-PCR was performed as described previously [87] (see S1 File). To examine miRNA expression in different regions of females, 28 day females were dissected into three parts including an anterior, ovary, and vitellarium. The miScript primers were Qiagen's proprietary primers. For semi-quantitative RT-PCR or stem-loop based qRT-PCR, a stem-loop based RT-PCR method was used to analyze miRNA expression (see S1 File). A stem-loop RT primer was used to reverse-transcribe mature miRNAs to cDNAs. Then, PCR was performed for miRNA abundance analysis. For semi-quantitative RT-PCR, the PCR products were analyzed on 2% agarose gels and images were captured under a UV light using a digital camera. The primers used for stem-loop based qRT-PCR and semi-quantitative RT-PCR are listed in S4 Table.
Argonaute antibody-based pull-down assay
Adult paired schistosomes (~150 mg, 28 day post-infection) were lysed in 500 μL lysis buffer containing 25 mM Tris (pH 7.4), 150 mM KCl, 0.5% NP-40, 2 mM EDTA, 1 M NaF, 0.5 M DTT and protease inhibitors (Roche) and were centrifuged at 10,000×g for 10 min at 4°C. The pull down assay was performed using a Dynabeads Protein G immunoprecipitation Kit (Invitrogen) according to the manufacturer’s instructions. We used three previously developed polyclonal antibodies to the three S. japonicum Ago proteins [60] and combined them for the pull-down assay. Briefly, approximate 25 mL of each anti-Ago serum was coupled to 1.5 mg Dynabeads. Then, 200 μL of worm lysate was incubated with the antiserum-coupled beads for 20 min at room temperature, and the Dynabeads were washed three times in 200 μL wash buffer provided by the kit. Finally, target antigens were eluted with the kit elution buffer. The elution was analyzed by SDS-PAGE electrophoresis and sliver staining as described in our previous study [10]. Western blot analyses were used to confirm the immunoprecipitation (IP) results.
Isolation of co-precipitated RNA and PCR amplification
RNA was isolated from the Argonuate IPs using TRIzol LS (Invitrogen) as described above, and the isolated RNA was reverse transcribed using Superscript II (Invitrogen) and the primer 5’ GCT GTC AAC GAT ACG CTA CGT AAC GGC ATG ACA GTG TTT TTT TTT TTT TTT TTT TTNN 3’, where N represents the random nucleotides G/C/A. The cDNAs were amplified using a specific forward primer designed for each miRNA seed sequence and 0.5 μM universal reverse primer (S5 Table), the PCR products were resolved on a 1.5% agarose gel, purified using an AxyPrep 96 PCR Clean Kit (Axygen), and cloned into pMD19-T vector (Takara). Over twenty recombinant clones for each miRNA were randomly selected for sequencing. To determine the enrichment of mRNA targets in the Argonaute pull-downs, five selected targets (GenBank accession numbers: AY233092.1, FN319623.1, FN32339.4, EU370927, AY815078.1) were evaluated by comparing the abundance of the RNA in Argonaute pulled down in the IPs to an IP control, total RNAs isolated from protein lysates, and the supernatant.
Bioinformatic analyses for miRNA targets
In silico prediction of miRNA targets was performed using RNAhybrid software (-b 1 -m 100000 -v 3 -u 3 -s 3utr_worm -e -19 -t gene.fa -q miRNA.fa). The sequences of the PCR products from the Argonaute IP analyses were characterized by using BLAST against S. japonicum nucleotide sequences in the NCBI GenBank collection (http://www.ncbi.nlm.nih.gov) and an E-value cutoff < 1e-20. Only the sequences annotated as protein coding were further characterized by miRNA:mRNA pairwise analyses using the online RNAhybrid program (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/). A miRNA:mRNA duplex with a minimum free energy (MFE) < = -19 kcal/mole was considered as a probable miRNA/mRNA target pair.
Vector construction
cDNA fragments corresponding to regions complementary to miRNAs (target genes) were PCR-amplified from S. japonicum cDNA and the PCR products were cloned into pCMV-Glu vector (Targeting Systems, USA) using standard molecular cloning methods. The primer pairs used for PCR amplification and the restriction enzyme sites are listed in S7 Table. The recombinant plasmids were confirmed by sequence analysis.
Cell transfection
HEK293T cells or Hela cells were cultured in 48 well plates containing Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% fetal bovine serum (Hyclone), 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) and incubated at 37°C in 5% CO2. Recombinant and control plasmids were transfected at 0.5 ng to 1.5 ng per well into cultured cells using Lipofectamine 2000 (Invitrogen) along with pGL3 (40 ng) for normalization. At 24h post transfection, the cells were further transfected with a miRNA mimic, miRNA inhibitor, or irrelevant miRNA (S8 Table) at the indicated concentrations using Lipofectamine 2000 (Invitrogen). The miRNA mimics, ant-sense miRNAs or scrambled miRNAs used were modified by 2`-O-methyl or 2`-O-methyl and phosphorothioate. The transfected cells were incubated for the indicated time prior to collection for dual luciferase assay as described below. Transfections were carried out in triplicate at least six times using two independent plasmid preparations.
Luciferase reporter assay
At 24–48 h post-transfection, luciferase activity was analyzed using a dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions. Relative reporter activity for transfected cells was obtained by normalization to either co-transfected firefly luciferase activity (pGl-3) or protein concentration using the Pierce BCA protein assay kit and Compat-Able protein assay preparation reagent set (Pierce).
In situ hybridization
In situ hybridization for determining miRNA and mRNA localization in S. japonicum was performed according to the method for S. mansoni WISH analysis with modifications [92]. The method is further described in S1 File and the LNA probes and the primers for probe preparation are listed in S10 Table.
Schistosome culture and electroporation
S. japonicum were collected from mice 24–28 days post-infection, females were separated and cultured in a 12-well flat bottom plate containing 2 mL complete RPMI-1640 media supplemented with 2 g/L glucose, 0.3 g/L l-glutamine, 2.0 g/L NaHCO3, 15% fetal bovine serum (heat inactivated), and 5% pen/strep (10,000 units penicillin and 10 mg/streptomycin in 0.9% NaCl) in a humidified 5% CO2 chamber at 37°C. miRNA inhibitors (antisense miRNA) and scrambled miRNA inhibitors (3 μg per experiment, chemically synthesized in Shanghai GenePharma, China) (S8 Table) were electroporated (125 V, 20 ms, 1 pulse in 200 μL RPMI 1640 media) into cultured schistosomes. Schistosomes were then transferred into a 12-well cell culture plates containing 2 mL fresh media. Worm mobility and survival were observed under an inverted microscope (Olympus, Japan) at the indicated times. The parasites were collected at 96 hr post electroporation for qRT-PCR analysis and confocal microscopy as described below.
qRT-PCR analysis of target gene transcripts in electroporated schistosomes
At 96 h post electroporation, RNA was isolated from worms using RNAiso plus (Takara) according to the manufacturer’s protocol except for an extended overnight precipitation in isopropanol at -80°C. First strand cDNA was produced from 100 ng RNA using a PrimeScript 1st Strand cDNA Synthesis Kit (Takara) with either an miRNA specific primer (2 μM, 0.5 μL) or random primer combined with Oligo d(T) as described above. PCR was then carried out using primers described in S9 Table. S. japonicum NADH (forward primer: CGA GGA CCT AAC AGC AGA GG; reverse primer: TCC GAA CGA ACT TTG AAT CC) was used as an internal control. The 2-ΔCt method was used to calculate relative expression [62].
Confocal microscopy of schistosome morphology
At 96 h post electroporation, the worms were preserved, stained, and mounted as described previously [93] and subjected to morphological examination using confocal microscopy (Nikon, Japan). Briefly, the worms were fixed in formalin (10%), alcohol (48%) and glacial acetic acid (2%), stained with carmine red, cleared in 0.5% hydrochloric alcohol solution, and preserved as whole mounts. Images were obtained with a Nikon CLSI laser confocal microscope (Nikon, Japan), using a 488 nm He/Ne laser. Z-Stacks were transformed into movies. Images in the Z-stacks was selected for pixel value analysis using ImageJ [94]. At least four female schistosomes were randomly selected for ImageJ analyses.
Supporting Information
Pairing of S. japonicum males and females usually occurs 15–18 days post-infection in the mammalian host, gametogenesis begins at 19–21 days post-infection, and male and female schistosomes begin to produce mature gametes at 22 days post-infection.
(PDF)
(A) Classification of small RNAs in 16-day old females (16F), 16-day old males (16M), 22-day old females (22F), 22-day old males (22M), 28-day old females (28F), 28-day old males (28M), and mixed males (M) and mixed females (F). (B) Size distribution of small RNAs in different S. japonicum stages. (C) Classification and percentage of S. japonicum small RNAs from different stages and sexes. Unannotated = small RNAs that map to the genome, but the genome regions are not annotated.
(PDF)
(A) Analysis of pull down products based on SDS-PAGE and silver staining. (B) Western blot analysis of the pull-downs.
(PDF)
(PDF)
(PDF)
Arrows indicate ovary in S. japonicum. Bars indicate 100 μm.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(A) Effect of miR-31 suppression on worm mortality in female schistosomes. (B) Effect of bantam suppression on worm mortality in female schistosomes. Data illustrate the mean and standard error derived from triplicate experiments including at least 30 female schistosomes.
(PDF)
The female schistosomes were electroporated with anti-miRNAs or scrambled anti-miRNAs and their effects on the levels of endogenous miRNA was determined by qRT-PCR at 4 days of post-electroporation. Data illustrate the mean and standard error derived from triplicate experiments. * means P ≤ 0.05 (student’s t test, miRNA inhibitor treatment vs scrambled inhibitor treatment).
(PDF)
The length and read number of the small RNA are indicated at the end of each small RNA sequence. The reads numbers are the sum of small RNA reads in all of the 8 libraries.
(PDF)
(PDF)
(XLSX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(AVI)
(AVI)
(AVI)
(AVI)
Acknowledgments
We thank Drs. Christoph G. Grevelding and David L. Williams for their comments on the manuscript.
Data Availability
All raw data with the small RNA deep sequencing has been submitted into GEO (accession number: GSE74654). All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by National Natural Science Foundation of China (http://www.nsfc.gov.cn/)(Grant No. 30901068), Science and Technology Commission of Shanghai Municipality of China (http://www.stcsm.gov.cn/)(Grant No.10410703400), Shanghai Talent Developing Foundation of China (http://www.21cnhr.gov.cn/) (Grant No.2009032). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gryseels B, Polman K, Clerinx J, Kestens L (2006) Human schistosomiasis. Lancet 368: 1106–1118. [DOI] [PubMed] [Google Scholar]
- 2. Mo AX, Agosti JM, Walson JL, Hall BF, Gordon L (2014) Schistosomiasis elimination strategies and potential role of a vaccine in achieving global health goals. Am J Trop Med Hyg 90: 54–60. 10.4269/ajtmh.13-0467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caffrey CR (2007) Chemotherapy of schistosomiasis: present and future. Curr Opin Chem Biol 11: 433–439. [DOI] [PubMed] [Google Scholar]
- 4. Caffrey CR, Secor WE (2011) Schistosomiasis: from drug deployment to drug development. Curr Opin Infect Dis 24: 410–417. 10.1097/QCO.0b013e328349156f [DOI] [PubMed] [Google Scholar]
- 5. Doenhoff MJ, Pica-Mattoccia L (2006) Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Review of Anti-Infective Therapy 4: 199–210. [DOI] [PubMed] [Google Scholar]
- 6. Severinghaus AE (1928) Sex studies on Schistosoma japonicum. Quarterly Journal of Microscopical Science s2: (284): 653. [Google Scholar]
- 7. Popiel I (1986) Male-stimulated female maturation in Schistosoma: A review. J Chem Ecol 12: 1745–1754. 10.1007/BF01022380 [DOI] [PubMed] [Google Scholar]
- 8. Kunz W (2001) Schistosome male-female interaction: induction of germ-cell differentiation. Trends Parasitol 17: 227–231. [DOI] [PubMed] [Google Scholar]
- 9. LoVerde PT (2002) Presidential address. Sex and schistosomes: an interesting biological interplay with control implications. J Parasitol 88: 3–13. [DOI] [PubMed] [Google Scholar]
- 10. Cheng GF, Lin JJ, Feng XG, Fu ZQ, Jin YM, et al. (2005) Proteomic analysis of differentially expressed proteins between the male and female worm of Schistosoma japonicum after pairing. Proteomics 5: 511–521. [DOI] [PubMed] [Google Scholar]
- 11. Pearce EJ, MacDonald AS (2002) The immunobiology of schistosomiasis. Nat Rev Immunol 2: 499–511. [DOI] [PubMed] [Google Scholar]
- 12. Cheng G, Luo R, Hu C, Lin J, Bai Z, et al. (2013) TiO2-based phosphoproteomic analysis of schistosomes: characterization of phosphorylated proteins in the different stages and sex of Schistosoma japonicum . J Proteome Res 12: 729–742. 10.1021/pr3007864 [DOI] [PubMed] [Google Scholar]
- 13. Popiel I, Basch PF (1984) Reproductive development of female Schistosoma mansoni (Digenea: Schistosomatidae) following bisexual pairing of worms and worm segments. J Exp Zool 232: 141–150. [DOI] [PubMed] [Google Scholar]
- 14. Basch PF (1988) Schistosoma mansoni: nucleic acid synthesis in immature females from single-sex infections, paired in vitro with intact males and male segments. Comp Biochem Physiol B 90: 389–392. [DOI] [PubMed] [Google Scholar]
- 15. Den Hollander JE, Erasmus DA (1985) Schistosoma mansoni: male stimulation and DNA synthesis by the female. Parasitology 91 (Pt 3): 449–457. [DOI] [PubMed] [Google Scholar]
- 16. Gupta BC, Basch PF (1987) The role of Schistosoma mansoni males in feeding and development of female worms. J Parasitol 73: 481–486. [PubMed] [Google Scholar]
- 17. LoVerde PT, Chen L (1991) Schistosome female reproductive development. Parasitol Today 7: 303–308. [DOI] [PubMed] [Google Scholar]
- 18. Galanti SE, Huang SC, Pearce EJ (2012) Cell death and reproductive regression in female Schistosoma mansoni . PLoS Negl Trop Dis 6: e1509 10.1371/journal.pntd.0001509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knobloch J, Kunz W, Grevelding CG (2002) Quantification of DNA synthesis in multicellular organisms by a combined DAPI and BrdU technique. Dev Growth Differ 44: 559–563. [DOI] [PubMed] [Google Scholar]
- 20. Zhou Y, Zheng H, Chen Y, Zhang L, Wang K, et al. (2009) The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature 460: 345–351. 10.1038/nature08140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu F, Lu J, Hu W, Wang SY, Cui SJ, et al. (2006) New perspectives on host-parasite interplay by comparative transcriptomic and proteomic analyses of Schistosoma japonicum . PLoS Pathog 2: e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moertel L, McManus DP, Piva TJ, Young L, McInnes RL, et al. (2006) Oligonucleotide microarray analysis of strain- and gender-associated gene expression in the human blood fluke, Schistosoma japonicum . Mol Cell Probes 20: 280–289. [DOI] [PubMed] [Google Scholar]
- 23. Cogswell AA, Kommer VP, Williams DL (2012) Transcriptional analysis of a unique set of genes involved in Schistosoma mansoni female reproductive biology. PLoS Negl Trop Dis 6: e1907 10.1371/journal.pntd.0001907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piao X, Cai P, Liu S, Hou N, Hao L, et al. (2011) Global expression analysis revealed novel gender-specific gene expression features in the blood fluke parasite Schistosoma japonicum . PLoS One 6: e18267 10.1371/journal.pone.0018267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. You H, Zhang W, Moertel L, McManus DP, Gobert GN (2009) Transcriptional profiles of adult male and female Schistosoma japonicum in response to insulin reveal increased expression of genes involved in growth and development. Int J Parasitol 39: 1551–1559. 10.1016/j.ijpara.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 26. Fitzpatrick JM, Hoffmann KF (2006) Dioecious Schistosoma mansoni express divergent gene repertoires regulated by pairing. Int J Parasitol 36: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 27. Fitzpatrick JM, Hirai Y, Hirai H, Hoffmann KF (2007) Schistosome egg production is dependent upon the activities of two developmentally regulated tyrosinases. FASEB J 21: 823–835. [DOI] [PubMed] [Google Scholar]
- 28. Wuhrer M, Koeleman CA, Fitzpatrick JM, Hoffmann KF, Deelder AM, et al. (2006) Gender-specific expression of complex-type N-glycans in schistosomes. Glycobiology 16: 991–1006. [DOI] [PubMed] [Google Scholar]
- 29. Waisberg M, Lobo FP, Cerqueira GC, Passos LK, Carvalho OS, et al. (2007) Microarray analysis of gene expression induced by sexual contact in Schistosoma mansoni . BMC Genomics 8: 181 [DOI] [PubMed] [Google Scholar]
- 30. Popiel I, Basch PF (1984) Putative polypeptide transfer from male to female Schistosoma mansoni . Mol Biochem Parasitol 11: 179–188. [DOI] [PubMed] [Google Scholar]
- 31. Chen LL, Rekosh DM, LoVerde PT (1992) Schistosoma mansoni p48 eggshell protein gene: characterization, developmentally regulated expression and comparison to the p14 eggshell protein gene. Mol Biochem Parasitol 52: 39–52. [DOI] [PubMed] [Google Scholar]
- 32. Gupta BC, Basch PF (1987) Evidence for transfer of a glycoprotein from male to female Schistosoma mansoni during pairing. J Parasitol 73: 674–675. [PubMed] [Google Scholar]
- 33. Grevelding CG, Sommer G, Kunz W (1997) Female-specific gene expression in Schistosoma mansoni is regulated by pairing. Parasitology 115 (Pt 6): 635–640. [DOI] [PubMed] [Google Scholar]
- 34. Siegel DA, Tracy JW (1989) Schistosoma mansoni: influence of the female parasite on glutathione biosynthesis in the male. Exp Parasitol 69: 116–124. [DOI] [PubMed] [Google Scholar]
- 35. Schussler P, Grevelding CG, Kunz W (1997) Identification of Ras, MAP kinases, and a GAP protein in Schistosoma mansoni by immunoblotting and their putative involvement in male-female interaction. Parasitology 115 (Pt 6): 629–634. [DOI] [PubMed] [Google Scholar]
- 36. Atkinson KH, Atkinson BG (1980) Biochemical basis for the continuous copulation of female Schistosoma mansoni . Nature 283: 478–479. [DOI] [PubMed] [Google Scholar]
- 37. Knobloch J, Kunz W, Grevelding CG (2006) Herbimycin A suppresses mitotic activity and egg production of female Schistosoma mansoni . Int J Parasitol 36: 1261–1272. [DOI] [PubMed] [Google Scholar]
- 38. Osman A, Niles EG, Verjovski-Almeida S, LoVerde PT (2006) Schistosoma mansoni TGF-beta receptor II: role in host ligand-induced regulation of a schistosome target gene. PLoS Pathogens 2: e54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freitas TC, Jung E, Pearce EJ (2007) TGF-beta signaling controls embryo development in the parasitic flatworm Schistosoma mansoni . PLoS Pathogens 3: e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. LoVerde PT, Osman A, Hinck A (2007) Schistosoma mansoni: TGF-beta signaling pathways. Experimental Parasitology 117: 304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buro C, Oliveira KC, Lu Z, Leutner S, Beckmann S, et al. (2013) Transcriptome analyses of inhibitor-treated schistosome females provide evidence for cooperating Src-kinase and TGFbeta receptor pathways controlling mitosis and eggshell formation. PLoS Pathog 9: e1003448 10.1371/journal.ppat.1003448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leutner S, Oliveira KC, Rotter B, Beckmann S, Buro C, et al. (2013) Combinatory microarray and SuperSAGE analyses identify pairing-dependently transcribed genes in Schistosoma mansoni males, including follistatin. PLoS Negl Trop Dis 7: e2532 10.1371/journal.pntd.0002532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beckmann S, Buro C, Dissous C, Hirzmann J, Grevelding CG (2010) The Syk kinase SmTK4 of Schistosoma mansoni is involved in the regulation of spermatogenesis and oogenesis. PLoS Pathog 6: e1000769 10.1371/journal.ppat.1000769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cai P, Piao X, Hao L, Liu S, Hou N, et al. (2013) A deep analysis of the small non-coding RNA population in Schistosoma japonicum eggs. PLoS One 8: e64003 10.1371/journal.pone.0064003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cai P, Hou N, Piao X, Liu S, Liu H, et al. (2011) Profiles of small non-coding RNAs in Schistosoma japonicum during development. PLoS Negl Trop Dis 5: e1256 10.1371/journal.pntd.0001256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Z, Xue X, Sun J, Luo R, Xu X, et al. (2010) An "in-depth" description of the small non-coding RNA population of Schistosoma japonicum schistosomulum. PLoS Negl Trop Dis 4: e596 10.1371/journal.pntd.0000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bobek LA, Rekosh DM, LoVerde PT (1991) Schistosoma japonicum: analysis of eggshell protein genes, their expression, and comparison with similar genes from other schistosomes. Exp Parasitol 72: 381–390. [DOI] [PubMed] [Google Scholar]
- 48. de Souza Gomes M, Muniyappa MK, Carvalho SG, Guerra-Sa R, Spillane C (2011) Genome-wide identification of novel microRNAs and their target genes in the human parasite Schistosoma mansoni . Genomics 98: 96–111. 10.1016/j.ygeno.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 49. Simoes MC, Lee J, Djikeng A, Cerqueira GC, Zerlotini A, et al. (2011) Identification of Schistosoma mansoni microRNAs. BMC Genomics 12: 47 10.1186/1471-2164-12-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marco A, Kozomara A, Hui JH, Emery AM, Rollinson D, et al. (2013) Sex-biased expression of microRNAs in Schistosoma mansoni . PLoS Negl Trop Dis 7: e2402 10.1371/journal.pntd.0002402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oliveira KC, Carvalho ML, Maracaja-Coutinho V, Kitajima JP, Verjovski-Almeida S (2011) Non-coding RNAs in schistosomes: an unexplored world. An Acad Bras Cienc 83: 673–694. [DOI] [PubMed] [Google Scholar]
- 52. Hao L, Cai P, Jiang N, Wang H, Chen Q (2010) Identification and characterization of microRNAs and endogenous siRNAs in Schistosoma japonicum . BMC Genomics 11: 55 10.1186/1471-2164-11-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang J, Hao P, Chen H, Hu W, Yan Q, et al. (2009) Genome-wide identification of Schistosoma japonicum microRNAs using a deep-sequencing approach. PLoS One 4: e8206 10.1371/journal.pone.0008206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xue X, Sun J, Zhang Q, Wang Z, Huang Y, et al. (2008) Identification and characterization of novel microRNAs from Schistosoma japonicum . PLoS One 3: e4034 10.1371/journal.pone.0004034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheng G, Jin Y (2012) MicroRNAs: Potentially important regulators for schistosome development and therapeutic targets against schistosomiasis. Parasitology 139: 669–679. 10.1017/S0031182011001855 [DOI] [PubMed] [Google Scholar]
- 56. Zhu L, Liu J, Cheng G (2014) Role of microRNAs in schistosomes and schistosomiasis. Front Cell Infect Microbiol 4: 165 10.3389/fcimb.2014.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Luo R, Xue X, Wang Z, Sun J, Zou Y, et al. (2010) Analysis and characterization of the genes encoding the Dicer and Argonaute proteins of Schistosoma japonicum . Parasit Vectors 3: 90 10.1186/1756-3305-3-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gomes MS, Cabral FJ, Jannotti-Passos LK, Carvalho O, Rodrigues V, et al. (2009) Preliminary analysis of miRNA pathway in Schistosoma mansoni . Parasitology International 58: 61–68. 10.1016/j.parint.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 59. Gomes MS, Cabral FJ, Jannotti-Passos LK, Carvalho O, Rodrigues V, et al. (2009) Preliminary analysis of miRNA pathway in Schistosoma mansoni . Parasitol Int 58: 61–68. 10.1016/j.parint.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 60. Chen J, Yang Y, Guo S, Peng J, Liu Z, et al. (2010) Molecular cloning and expression profiles of Argonaute proteins in Schistosoma japonicum . Parasitol Res 107: 889–899. 10.1007/s00436-010-1946-3 [DOI] [PubMed] [Google Scholar]
- 61. Wang J, Czech B, Crunk A, Wallace A, Mitreva M, et al. (2011) Deep small RNA sequencing from the nematode Ascaris reveals conservation, functional diversification, and novel developmental profiles. Genome Res 21: 1462–1477. 10.1101/gr.121426.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 63. Karginov FV, Conaco C, Xuan Z, Schmidt BH, Parker JS, et al. (2007) A biochemical approach to identifying microRNA targets. Proc Natl Acad Sci U S A 104: 19291–19296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jaskiewicz L, Bilen B, Hausser J, Zavolan M (2012) Argonaute CLIP—a method to identify in vivo targets of miRNAs. Methods 58: 106–112. 10.1016/j.ymeth.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 65. Knobloch J, Beckmann S, Burmeister C, Quack T, Grevelding CG (2007) Tyrosine kinase and cooperative TGFbeta signaling in the reproductive organs of Schistosoma mansoni . Exp Parasitol 117: 318–336. [DOI] [PubMed] [Google Scholar]
- 66. LoVerde PT, Osman A, Hinck A (2007) Schistosoma mansoni: TGF-beta signaling pathways. Exp Parasitol 117: 304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Freitas TC, Jung E, Pearce EJ (2007) TGF-beta signaling controls embryo development in the parasitic flatworm Schistosoma mansoni . PLoS Pathog 3: e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beckmann S, Quack T, Burmeister C, Buro C, Long T, et al. (2010) Schistosoma mansoni: signal transduction processes during the development of the reproductive organs. Parasitology 137: 497–520. 10.1017/S0031182010000053 [DOI] [PubMed] [Google Scholar]
- 69. Hu W, Yan Q, Shen DK, Liu F, Zhu ZD, et al. (2003) Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nat Genet 35: 139–147. [DOI] [PubMed] [Google Scholar]
- 70. Verjovski-Almeida S, Leite LC, Dias-Neto E, Menck CF, Wilson RA (2004) Schistosome transcriptome: insights and perspectives for functional genomics. Trends Parasitol 20: 304–308. [DOI] [PubMed] [Google Scholar]
- 71. Protasio AV, Tsai IJ, Babbage A, Nichol S, Hunt M, et al. (2012) A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni . PLoS Negl Trop Dis 6: e1455 10.1371/journal.pntd.0001455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Copeland CS, Marz M, Rose D, Hertel J, Brindley PJ, et al. (2009) Homology-based annotation of non-coding RNAs in the genomes of Schistosoma mansoni and Schistosoma japonicum . BMC Genomics 10: 464 10.1186/1471-2164-10-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liang S, Knight M, Jolly ER (2013) Polyethyleneimine mediated DNA transfection in schistosome parasites and regulation of the WNT signaling pathway by a dominant-negative SmMef2. PLoS Negl Trop Dis 7: e2332 10.1371/journal.pntd.0002332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Oliveira KC, Carvalho ML, Verjovski-Almeida S, LoVerde PT (2012) Effect of human TGF-beta on the gene expression profile of Schistosoma mansoni adult worms. Mol Biochem Parasitol 183: 132–139. 10.1016/j.molbiopara.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Winklbauer R, Medina A, Swain RK, Steinbeisser H (2001) Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature 413: 856–860. [DOI] [PubMed] [Google Scholar]
- 76. Silvie O, Goetz K, Matuschewski K (2008) A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog 4: e1000086 10.1371/journal.ppat.1000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lairson LL, Henrissat B, Davies GJ, Withers SG (2008) Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem 77: 521–555. 10.1146/annurev.biochem.76.061005.092322 [DOI] [PubMed] [Google Scholar]
- 78. Zhang L, Zhang Y, Hagen KG (2008) A mucin-type O-glycosyltransferase modulates cell adhesion during Drosophila development. J Biol Chem 283: 34076–34086. 10.1074/jbc.M804267200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shin C, Manley JL (2002) The SR protein SRp38 represses splicing in M phase cells. Cell 111: 407–417. [DOI] [PubMed] [Google Scholar]
- 80. Yang L, Embree LJ, Hickstein DD (2000) TLS-ERG leukemia fusion protein inhibits RNA splicing mediated by serine-arginine proteins. Mol Cell Biol 20: 3345–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM (2003) bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113: 25–36. [DOI] [PubMed] [Google Scholar]
- 82. Nolo R, Morrison CM, Tao C, Zhang X, Halder G (2006) The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr Biol 16: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 83. Thompson BJ, Cohen SM (2006) The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell 126: 767–774. [DOI] [PubMed] [Google Scholar]
- 84. Herranz H, Perez L, Martin FA, Milan M (2008) A Wingless and Notch double-repression mechanism regulates G1-S transition in the Drosophila wing. EMBO J 27: 1633–1645. 10.1038/emboj.2008.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Oh H, Irvine KD (2011) Cooperative regulation of growth by Yorkie and Mad through bantam. Dev Cell 20: 109–122. 10.1016/j.devcel.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Herranz H, Hong X, Cohen SM (2012) Mutual repression by bantam miRNA and Capicua links the EGFR/MAPK and Hippo pathways in growth control. Curr Biol 22: 651–657. 10.1016/j.cub.2012.02.050 [DOI] [PubMed] [Google Scholar]
- 87. Cheng G, Luo R, Hu C, Cao J, Jin Y (2013) Deep sequencing-based identification of pathogen-specific microRNAs in the plasma of rabbits infected with Schistosoma japonicum . Parasitology 140: 1751–1761. 10.1017/S0031182013000917 [DOI] [PubMed] [Google Scholar]
- 88. Beall MJ, McGonigle S, Pearce EJ (2000) Functional conservation of Schistosoma mansoni Smads in TGF-beta signaling. Mol Biochem Parasitol 111: 131–142. [DOI] [PubMed] [Google Scholar]
- 89. Carlo JM, Osman A, Niles EG, Wu W, Fantappie MR, et al. (2007) Identification and characterization of an R-Smad ortholog (SmSmad1B) from Schistosoma mansoni. FEBS J 274: 4075–4093. [DOI] [PubMed] [Google Scholar]
- 90. Hahnel S, Quack T, Parker-Manuel SJ, Lu Z, Vanderstraete M, et al. (2014) Gonad RNA-specific qRT-PCR analyses identify genes with potential functions in schistosome reproduction such as SmFz1 and SmFGFRs. Front Genet 5: 170 10.3389/fgene.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang Z, Xue X, Sun J, Luo R, Xu X, et al. (2010) An "in-depth" description of the small non-coding RNA population of Schistosoma japonicum schistosomulum. PLoS Neglected Tropical Diseases 4: e596 10.1371/journal.pntd.0000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cogswell AA, Collins JJ 3rd, Newmark PA, Williams DL (2011) Whole mount in situ hybridization methodology for Schistosoma mansoni . Mol Biochem Parasitol 178: 46–50. 10.1016/j.molbiopara.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang WN, Zhang P, Liu M, Ren CP, Jia XM, et al. (2012) Worm morphology of Schistosoma japonicum using confocal laser scanning microscopy. J Helminthol 86: 317–322. 10.1017/S0022149X11000447 [DOI] [PubMed] [Google Scholar]
- 94. Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pairing of S. japonicum males and females usually occurs 15–18 days post-infection in the mammalian host, gametogenesis begins at 19–21 days post-infection, and male and female schistosomes begin to produce mature gametes at 22 days post-infection.
(PDF)
(A) Classification of small RNAs in 16-day old females (16F), 16-day old males (16M), 22-day old females (22F), 22-day old males (22M), 28-day old females (28F), 28-day old males (28M), and mixed males (M) and mixed females (F). (B) Size distribution of small RNAs in different S. japonicum stages. (C) Classification and percentage of S. japonicum small RNAs from different stages and sexes. Unannotated = small RNAs that map to the genome, but the genome regions are not annotated.
(PDF)
(A) Analysis of pull down products based on SDS-PAGE and silver staining. (B) Western blot analysis of the pull-downs.
(PDF)
(PDF)
(PDF)
Arrows indicate ovary in S. japonicum. Bars indicate 100 μm.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(A) Effect of miR-31 suppression on worm mortality in female schistosomes. (B) Effect of bantam suppression on worm mortality in female schistosomes. Data illustrate the mean and standard error derived from triplicate experiments including at least 30 female schistosomes.
(PDF)
The female schistosomes were electroporated with anti-miRNAs or scrambled anti-miRNAs and their effects on the levels of endogenous miRNA was determined by qRT-PCR at 4 days of post-electroporation. Data illustrate the mean and standard error derived from triplicate experiments. * means P ≤ 0.05 (student’s t test, miRNA inhibitor treatment vs scrambled inhibitor treatment).
(PDF)
The length and read number of the small RNA are indicated at the end of each small RNA sequence. The reads numbers are the sum of small RNA reads in all of the 8 libraries.
(PDF)
(PDF)
(XLSX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(AVI)
(AVI)
(AVI)
(AVI)
Data Availability Statement
All raw data with the small RNA deep sequencing has been submitted into GEO (accession number: GSE74654). All other relevant data are within the paper and its Supporting Information files.