Figure S4.
Cholera Toxin and Dynein Staining on Late Phagosomes, Appearance of Dynein in DRM Fraction, and Effect of MβCD and LPG on Proteins Associated with Late Phagosomes, Related to Figures 3, 4, and 5
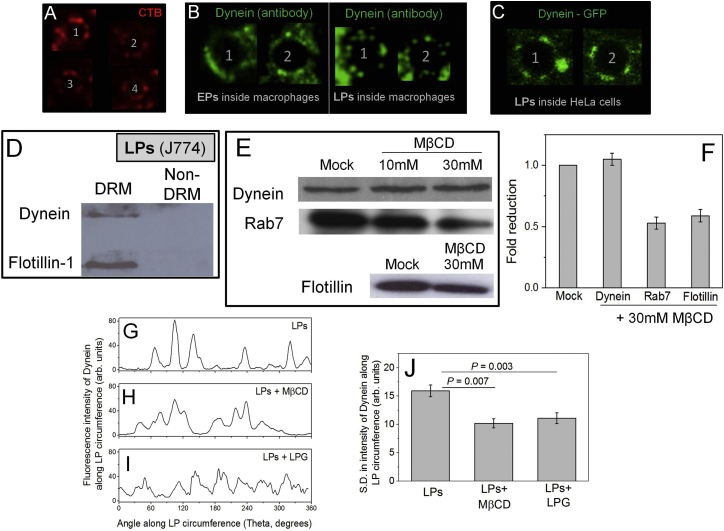
(A) LPs (numbered 1-4) inside J774 cells were stained against Alexa Fluor 594 conjugated Cholera Toxin Subunit B (CTB) and visualized in a confocal microscope. Staining around the LP circumference is seen, supporting the presence of cholesterol-rich microdomains/lipid rafts on LPs. LP diameter = 2 microns.
(B) Immunofluorescence staining of EPs (left panel) and LPs (right panel) against dynein inside mouse macrophage cells. The staining on EP circumference is more uniform compared to the punctate staining on LPs. Two EPs from different cells (marked 1,2) and two LPs from different cells (marked 1,2) are shown. EP and LP diameter = 2 microns.
(C) Staining of two LPs (marked 1,2) inside HeLa cells expressing Dynein-GFP (see text). No antibody is used. Dynein again appears as puncta on the LP surface. LP diameter = 2 microns.
(D) LPs purified from J774 cells were used to isolate detergent resistant membrane (DRM) and non-DRM fractions. Western blotting was performed to probe for presence of dynein intermediate chain and flotilllin. Both proteins are detected in the DRM fraction, but not detected in the non-DRM (soluble) fraction. This experiment was repeated thrice with similar results.
(E) Western blots against dynein, Rab7 and flotillin-1 as a function of MβCD concentration on LPs purified from J774 cells.
(F) Quantification of western blot intensities in Figure S4-E shows that there is no reduction in the amount of dynein upon MβCD treatment, but ∼50% reduction in Rab7 and flotillin-1. Error bars are SEMs. Dynein has a secondary binding site to late endosomes/lysosomes via the dynein light intermediate chain (LIC1) that is independent of Rab7-RILP-dynactin. It is therefore possible that dynein is retained through this mechanism even after cholesterol and Rab7 are lost after MβCD treatment.
(G–I) Fluorescence intensity profile along the circumference of an untreated LP, an MβCD-treated LP and an LPG-treated LP. Sharp peaks for dynein staining on untreated LPs are replaced by broader features after MβCD and LPG treatment.
(J) Standard deviation (SD) of fluorescence intensity profile measured for multiple LPs along their circumference (10 each of untreated, MβCD treated and LPG treated LPs used). SD was reduced significantly after MβCD and LPG treatment, suggesting that the clustered organization of dynein was replaced by a more uniform distribution. This was also verified by a cross correlation analysis of these intensity profiles. A more rigorous analysis of the disruption in clustering was not attempted because of the diffraction limited nature of these images.