Abstract
Evidence from patients with amnesia suggests that recognition memory span tasks engage both long-term memory (i.e., secondary memory) processes mediated by the diencephalic-medial temporal lobe memory system and working memory processes mediated by fronto-striatal systems. Thus, the recognition memory span task may be particularly effective for detecting memory deficits in disorders that disrupt both memory systems. The presence of unique pathology in fronto-striatal circuits in Dementia with Lewy Bodies (DLB) compared to AD suggests that performance on the recognition memory span task might be differentially affected in the two disorders even though they have quantitatively similar deficits in secondary memory. In the present study, patients with autopsy-confirmed DLB or AD, and normal control (NC) participants, were tested on separate recognition memory span tasks that required them to retain increasing amounts of verbal, spatial, or visual object (i.e., faces) information across trials. Results showed that recognition memory spans for verbal and spatial stimuli, but not face stimuli, were lower in patients with DLB than in those with AD, and more impaired relative to NC performance. This was despite similar deficits in the two patient groups on independent measures of secondary memory such as the total number of words recalled from Long-Term Storage on the Buschke Selective Reminding Test. The disproportionate vulnerability of recognition memory span task performance in DLB compared to AD may be due to greater fronto-striatal involvement in DLB and a corresponding decrement in cooperative interaction between working memory and secondary memory processes. Assessment of recognition memory span may contribute to the ability to distinguish between DLB and AD relatively early in the course of disease.
Keywords: Working Memory, Secondary Memory, Cognition, Dementia with Lewy Bodies, Alzheimer’s Disease
1. Introduction
Several studies have shown that mildly-demented patients with Alzheimer’s disease (AD) are impaired on recognition memory span tasks that require them to retain increasing amounts of verbal, spatial, or visual object information across trials (Moss et al., 1986; Salmon et al., 1989). In these tasks, a single item is presented on the initial trial, a second item is added on the next trial, a third item is added on the next trial, and so on, with instructions on each trial to identify the new item. The number of items increases until an error is made. Recognition memory span is the number of items identified prior to the first error. Unlike traditional span tasks (e.g., digit span) that rely primarily on working memory processes mediated by fronto-striatal circuits (Frank et al., 2001), the recognition memory span task additionally engages long-term memory (i.e., secondary memory) processes mediated by the diencephalic-medial temporal lobe memory system (Squire, 1992). Thus, patients with circumscribed amnesia secondary to diencephalic damage (i.e., Wernicke-Korsakoff’s disase; Moss et al., 1986) or bilateral hippocampal damage (Levy et al., 2003) are impaired on recognition span tasks across a wide variety of stimuli (e.g., words, spatial locations, faces, colors, odors). While patients with amnesia are able to retain a certain number of items in working memory over the first few trials, their performance falters when working memory capacity is exceeded and they must rely on hippocampus-dependent secondary memory to perform the task (Levy et al., 2003). The recognition memory span deficit of patients with AD is consistent with these findings given their early hippocampal involvement (Braak and Braak, 1991; West et al., 1994) and prominent secondary memory deficit (for review, see Salmon and Bondi, 2009).
Previous neuroimaging findings in neurologically intact populations suggest that episodic memory encoding that also places high demands on working memory, such as in a recognition memory span test, may elicit cooperative interaction between medial temporal lobe and fronto-striatal memory systems that mediate secondary and working memory respectively (Sadeh et al., 2011). To the degree that optimal performance on the recognition memory span task requires effective interaction between working memory and secondary memory processes, the task should be particularly sensitive to disorders that disrupt both memory systems. One such disorder is Dementia with Lewy bodies (DLB). DLB is a progressive neurodegenerative disorder that presents as a dementia syndrome similar to AD along with an increased likelihood of mild Parkinsonism (e.g., rigidity, bradykinesia, masked facies), well-formed visual hallucinations, and fluctuations in cognition or alertness (McKeith et al., 1996; 2005). DLB is characterized by cell loss and Lewy body deposition in brain stem nuclei (e.g., substantia nigra, locus ceuruleus) as in Parkinson’s disease (PD), and by diffuse deposition of Lewy bodies in limbic regions (e.g., hippocampus, amygdala) and the neocortex (Harding and Holliday, 2001). Concomitant AD pathology (i.e., neuritic plaques, neurofibrillary tangles) is also frequently present in a typical limbic/neocortical distribution (Hansen et al., 1993; Harding and Holliday, 2001; Horimoto et al., 2003; Tsuboi and Dickson, 2005).
Memory dysfunction is often an early and prominent clinical feature of DLB (Ballard et al., 1996; Salmon et al., 1996; Walker et al., 1997; Connor et al., 1998; Shimomura et al., 1998; Heyman et al., 1999; Calderon et al., 2001). A study comparing mildly demented patients with autopsy-confirmed DLB (all with concomitant AD pathology) or “pure” AD (i.e., with no Lewy body pathology) on the California Verbal Learning Test and the WMS-R Logical Memory Test (Hamilton et al., 2004) showed that the groups were equally impaired in their ability to recall new verbal information. This secondary memory deficit is consistent with the medial temporal lobe pathology common to these disorders. While both groups were also impaired on delayed recognition of verbal information, these deficits were smaller in patients with DLB than in those with AD, suggesting that the medial temporal lobe damage may be less severe in DLB than in AD (Hansen et al., 1991). However, retrieval deficits associated with pathology in fronto-striatal circuits (Scimeca and Badre, 2012) may contribute to the secondary memory impairment of DLB making free recall appear as severe as in AD (Hamilton et al., 2004).
The presence of unique pathology in fronto-striatal circuits in DLB compared to AD suggests that performance on the recognition memory span task might be differentially affected in the two disorders. Given the important role of fronto-striatal circuits in working memory (Frank et al., 2001), the dysfunction of these circuits in DLB should cause a greater deficit in working memory in DLB than in AD. Previous studies with clinically-defined cohorts have indeed found greater working memory impairment in DLB than in AD (Sahgal et al., 1995). To the extent that recognition memory span performance depends on the effective interaction between working memory and secondary memory systems, the disruption of both memory systems in patients with DLB could lead to significantly worse performance than in patients with AD despite comparable deficits in secondary memory.
The present study examined this possibility by comparing recognition memory span in patients who at the time of testing demonstrated a mild dementia and were later diagnosed at autopsy as having AD or DLB. Given that patients with DLB have disproportionately severe visuospatial deficits compared to patients with AD (for review, see Metzler-Baddeley, 2007), recognition span was determined in three stimulus modalities (spatial, verbal and faces). The recognition memory span task is better suited for this manipulation than traditional span tasks (e.g., Wechsler Memory Scale digit span, spatial span or symbol span) since different stimulus modalities can be assessed using virtually identical procedures and behavioral requirements. Comparison of recognition span with verbal and non-verbal stimuli is important to determine whether the disparity in visuospatial deficits in DLB and AD extends to differences in their memory performance as well, or whether any recognition span deficits displayed by patients with DLB can be attributed to a general memory deficit per se rather than secondary to visuospatial impairment.
To insure that groups demonstrated comparable secondary memory deficits, patients were also administered the Selective Reminding Test (SRT; Buschke, 1973; Buschke and Fuld, 1974), a multi-trial verbal list learning task sensitive to deficits in secondary memory. The SRT distinguishes between recall from long-term and short-term storage by only re-presenting from trial-to-trial those words that were not recalled on the immediately preceding trial. Words remembered immediately after their re-presentation are considered to be recalled from short-term storage (i.e. immediate memory), whereas those remembered despite not being re-presented are considered to be recalled from long-term storage (i.e., secondary memory). Comparison of recognition memory span in the context of this independent measure of secondary memory might elucidate differences in recognition span capacity in DLB and AD. If secondary memory is impaired to the same degree in DLB and AD, then differences in recognition memory span would suggest that this task is assessing the additional disruption of working memory processes in DLB.
2. Materials and Methods
2.1 Participants
Patients with dementia who were eventually confirmed at autopsy to have DLB (n=14) or AD (n=14) were included in the present study. All patients participated in the University of California, San Diego (UCSD) Alzheimer’s Disease Research Center (ADRC), through which they received yearly physical, neurologic, and neuropsychological assessments. Eligible participants met the following inclusion criteria: 1) autopsy revealed no significant pathological process (e.g., hippocampal sclerosis, infarct with a clinical history of stroke, other significant neurovascular pathology likely to contribute to dementia) other than DLB or AD, 2) the Recognition Span Test and the Buschke Selective Reminding Test had been completed at one of the annual evaluations, and 3) they scored at least 14 on the Mini-Mental State Examination (MMSE) at the evaluation that included the Recognition Span Test. At the time of testing on the Recognition Span Test all patients carried a clinical diagnosis of dementia. A group of cognitively-healthy elderly individuals (n=25) who served as normal controls (NC) in the UCSD ADRC and completed the Recognition Span Test at one of their annual evaluations was included in the present study for comparison to the patient groups.
The patient and control groups did not differ significantly in age (F(2,50)=0.40; p=.67) or education (F(2,50)=1.23; p=.30) (see Table 1) at the time of testing. As expected, the groups differed in scores achieved on the MMSE (F(2,50)=32.97; p<.001. Post-hoc comparisons (Tukey’s Least Significant Difference test) showed that patients with DLB (p<.05) and patients with AD (p<.05) scored significantly lower than NC participants but did not differ from each other.
Table 1.
Mean age, years of education, and Mini-Mental State Exam (MMSE) scores of the Normal Control (NC) participants and patients with Dementia with Lewy Bodies (DLB) or Alzheimer’s Disease (AD). (Standard deviations are shown in parentheses).
| NC (n=25) | DLB (n=14) | AD (n=14) | |
|---|---|---|---|
| Age (years) | 72.5 (6.4) | 73.1 (6.7) | 71.0 (5.9) |
| Education (years) | 13.7 (2.8) | 12.9 (3.0) | 12.3 (2.6) |
| MMSE | 29.0 (0.9) | 21.1 (4.8) | 21.5 (4.6) |
Only a few DLB and AD patients were using psychoactive medications at the time of testing. One patient with DLB was using antidepressant medication (sinequan) and 1 was using sleep medication (Halcion). One patient with AD was using antidepressant medication (desipramine) and three were using anti-psychotic medication (1 on haldol, 2 on mellaril).
2.2 Procedure
Participants were tested individually in a quiet, well-lit room. The Recognition Span Test (Moss et al., 1986) and the Buschke Selective Reminding Test (BSRT; Buschke, 1973; Buschke and Fuld, 1974) were administered in the same session by the same examiner. The BSRT always preceded the Recognition Span Test and the two tests were separated by approximately one-hour of unrelated testing. Rest breaks were allowed as needed. The tests did not share stimulus materials (i.e. no overlapping words). Words in the BSRT were presented aurally and those in the verbal condition of the Recognition Span Test were presented visually. The modified Wisconsin Card Sorting Test (Nelson, 1976) was also administered to all participants during the test session as an independent assessment of the integrity of fronto-striatal circuits.
The research protocol was reviewed and approved by the human subjects review board at the University of California, San Diego. Informed consent to participate in the present investigation was obtained at the point of entry into the longitudinal study from all patients or their caregivers consistent with California State law. Informed consent for autopsy was obtained at the time of death from the next of kin.
2.3 Recognition Span Test
The stimulus materials were presented on a black test board (61 cm × 46 cm) upon which were mounted 30 yellow dots arranged in five rows of six dots each. Adjacent dots, each 12.5 mm in diameter, were separated by 9 cm, center to center. The dots served as targets for the positioning of the stimuli. A 61 cm × 48 cm, black, opaque sliding door was mounted perpendicular to the front of the test board so that placement of the stimuli could be shielded from the participant’s view.
The stimuli consisted of brown plastic disks, 5.08 cm in diameter, upon which different target materials could be mounted. Three sets of 14 disks were used with each set representing a different stimulus condition. In the spatial condition, 14 identical plain brown disks were used. In the verbal condition, each of the 14 disks carried a different five-letter word. The words were low imagery nouns and verbs (i.e., MONTH, START) that were of moderate to high frequency according to the Thorndike-Lorge word frequency count. In the faces condition, each of the 14 disks carried a different 2.54 cm × 3.81 cm photograph of a male face from a military academy yearbook. Since clothing, hair length, and general facial expression of the men pictured were similar, the configuration of the individual’s facial features was the most salient difference between the photographs.
The participant was seated across from the examiner with the test board centered on a table between them. The apparatus was positioned with the sliding door between the test board and the participant so that the participant could not see the board when the door was closed. The spatial, verbal, and faces conditions were presented, in that order, to all participants.
2.3.1 Spatial Condition
With the participant viewing the empty test board the examiner began by saying: “I am going to place one of these blank disks on the board. I want you to look at the disk and after a few seconds, I am going to close the door, covering the board.” The examiner then placed one disk at a predetermined location on the board and 15 sec later closed the door with the following instructions: “When I open the door another disk will be on the board. I want you to point to the new disk – the one that was not there before.” While the board was covered a second blank disk was placed at a predetermined location on the board. The first disk remained in its original location. Following a 10 sec delay interval, the door was opened and the examiner said: “Look over the board and point to the new disk.” The participant was allowed 10 sec to make a choice after which he or she was encouraged to choose, guessing if necessary. After each correct choice the participant was allowed to study all of the disks on the board for 5 sec, then the door was closed, a new disk was added, old disks remained in their original locations, and the procedure was repeated. A test series was discontinued after the first error or after all 14 disks had been presented. In the spatial and subsequent test conditions, recognition span was determined on two consecutive test series. The first series of each stimulus condition was preceded by a single practice trial to ensure that the participant understood the instructions of the task.
2.3.2 Verbal Condition
The procedure for administering the verbal condition was identical to that of the spatial with the following three exceptions. First, the examiner began the test by placing all 14 disks on the board and asking the participant to read each word aloud. Second, the examiner moved the disks randomly around the board during the delay interval of each trial in order to eliminate spatial cues. One disk was always presented in the location that had been chosen on the previous trial, and one disk was always placed in a location adjacent to the new disk. The following instructions were added: “This time when I cover the board, I am going to move all of the disks around so that their position cannot help you identify the new disk. When I open the door look at all of the disks carefully and then point to the new disk.” Third, a new disk was added to the board in the verbal condition even if the previous trial response was incorrect. Thus, all participants saw all 14 words on both test series. This procedure allowed assessment of free recall of the words used in the task. After completion of the second and last recognition span test series, the examiner waited 15 sec and then asked the participant to recall as many of the words used in the task as possible. The examiner then engaged the participant in conversation unrelated to the task for 2 min, after which the participant was asked to recall the words again. The participant was allowed approximately 1 min for each recall attempt.
2.3.3 Faces Condition
The procedure for administering the faces condition was identical to that of the verbal condition except that each test series was terminated after the first error. No initial exposure to the faces was provided, nor was an attempt at recall required.
2.4 Selective Reminding Test (SRT)
On the initial trial of this verbal list learning task, participants were read 10 unrelated words at a rate of one word every 2 sec and then asked to recall the entire list. On the second trial, the participant was only read those words they failed to recall on the first trial (i.e., selectively reminded) and again asked to recall the entire list. This procedure of presenting only those words that were not recalled on the preceding trial and asking for recall of the entire list was followed for a total of six trials. An item was considered recalled from short-term storage (STS) on each trial if it was recalled only immediately after its presentation (i.e., after it had just been “reminded”). An item was considered retrieved from long-term storage (LTS) when it was recalled on a trial in which it was not re-presented. Total recall from STS and LTS were determined by summing across the 6 learning trials. Immediately after the six learning trials, a two-alternative forced-choice recognition test for the 10 words was presented.
2.5 Modified Wisconsin Card Sorting Test (mWCST)
Participants were asked to sort 48 response cards on which are printed one to four symbols (triangle, star, cross, or circle) in one of four colors (red, green, yellow, or blue). Sorting is based on four stimulus cards that contain one red triangle, two green stars, three yellow crosses, or four blue circles, respectively, arranged in a row if front of the participant. Each response card matches three of the stimulus cards in terms of one, and only one, attribute and has no attribute in common with the fourth stimulus card. Participants must place each response card, one by one, below the appropriate stimulus card based on a matching principle that is in effect (i.e., color, symbol or number). The participant must deduce the matching principle based on the pattern of the examiner’s verbal feedback on whether the placement is correct or incorrect. Each principle remains in effect until six correct placements in a row are achieved. At that point, the participant is told that a new sorting principle is in effect (but not the nature of the principle). The test continues until six sorts (principles) are achieved or until all 48 response cards have been sorted. The number of sorts (principles) achieved and the numbers of perseverative and non-perseverative errors committed are recorded.
2.6 Neuropathologic Examination
Autopsy was performed within 12 hours of death using a previously described protocol (Terry et al., 1981). Briefly, the left hemibrain was fixed by immersion in 10% formalin for 5–7 days. Paraffin-embedded blocks from mid-frontal, rostral superior temporal, and inferior parietal neocortex, hippocampus, entorhinal cortex, basal ganglia/substantia innominata, mesencephalon, and pons were cut at 7 μm thickness for hematoxylin-eosin (H & E) and thioflavin-S stains. Total plaques, neuritic plaques, and neurofibrillary tangle counts were determined by viewing the thioflavin-S stains under polarized light. A Braak stage for neurofibrillary pathology was obtained for each case using previously detailed methods (Hansen and Samuel, 1997).
DLB cases met consensus criteria for the pathologic diagnosis of DLB based on hematoxylin-eosin (H & E) staining and antiubiquitin immunostaining (McKeith et al., 1996), and anti-α-synuclein immunostaining (McKeith et al., 2005). Cases were only construed as DLB if Lewy bodies were found in the locus ceruleus, substantia nigra, and/or nucleus basalis of Meynert, as well as in the neocortex. Because all cases categorized as DLB had neocortical as well as brainstem Lewy bodies, they all fell into either the limbic (transitional) or neocortical categories proposed in the 1996 consensus guidelines for the pathologic diagnosis of DLB (McKeith et al., 1996). Furthermore, all DLB cases were neocortical stage 5 or 6 according to the proposed Lewy-body based staging of brain pathology related to sporadic Parkinson’s disease (Braak et al., 2003). Cases were not classified as DLB if Lewy bodies were only found in the amygdala (McKeith et al., 2005).
The neuropathologic diagnosis of AD was based on both NIA-Reagan (1997) and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD; Mirra et al., 1991) criteria. Of the DLB patients, the likelihood that dementia was caused by AD was high in 36% (8/22), intermediate in 27% (6/22), and low in 27% (6/22) according to NIA-Reagan criteria. Based on CERAD criteria, the majority of DLB patients also had definite [68% (15/22)] or probable [27% (6/22)] AD. Overall, 95% of the DLB patients had concomitant AD by either NIA-Reagan or CERAD criteria and would conform to what Hansen and colleagues (1990) called Lewy Body Variant of AD. One DLB patient did not meet either NIA-Reagan or CERAD criteria for AD and would conform to what Hansen and colleagues (1990) previously called Diffuse Lewy Body Disease. The majority (91%) of the AD patients met both NIA-Reagan criteria for high likelihood that dementia is caused by AD and CERAD criteria for definite AD. The remaining four patients met criteria either for “high likelihood” or for definite AD. None of the AD cases had Lewy bodies recognized in the neocortex or pigmented brainstem nuclei where they are readily apparent with H & E stain and where they would appear prior to neocortical involvement, except in “amygdala-only” cases (Rub et al., 2002; Braak et al., 2003).
3. Results
3.1 Neuropathologic Findings
Brain weight at post-mortem did not differ significantly between the DLB (mean=1048.6; s.d.=120.7) and AD (mean=1138.3; s.d.=199.2) patients (t(25)=1.43; p=.17). All DLB patients satisfied criteria for diffuse neocortical type (McKeith et al., 2005). The AD Braak stage of the patients with DLB (mean = 3.5 ± 2.1) was significantly lower than that of the patients with AD (mean = 5.6 ± 0.6; χ2 (5) = 15.56, p = 0.008). The mean number of neuritic plaques and neurofibrillary tangles in the hippocampus and selected mid-frontal, inferior parietal and superior temporal neocortical regions for each group are shown in Table 2. Patients with DLB had significantly fewer neurofibrillary tangles than patients with AD in the hippocampus (t(25)=2.88; p=.008), inferior parietal cortex (t(26)=3.44; p=.002) and superior temporal cortex (t(25)=2.86; p=.009), but not in the mid-frontal cortex (t(25)=1.97; p=.06). Patients with DLB had significantly fewer neuritic plaques than patients with AD in the mid-frontal cortex (t(24)=3.64; p=.001), inferior parietal cortex (t(26)=2.56; p=.017) and superior temporal cortex (t(25)=3.29; p=.003), but not in the hippocampus (t(25)=1.11; p=.23).
Table 2.
The mean number of neuritic plaques and neurofibrillary tangles in the hippocampus and selected mid-frontal, inferior parietal, and superior temporal neocortical regions in patients with Dementia with Lewy Bodies and patients with Alzheimer’s Disease. (Standard deviations are shown in parentheses).
| Dementia with Lewy Bodies (n=14) | Alzheimer’s Disease (n=14) | |||
|---|---|---|---|---|
| Plaques | Tangles | Plaques | Tangles | |
| Hippocampus | 8.86 (7.04) | 5.71 (8.40) | 12.00 (7.66) | 21.38 (18.39) |
| Mid-Frontal | 32.46 (14.98) | 1.08 (3.88) | 48.38 (4.96) | 3.79 (3.24) |
| Inferior Parietal | 34.71 (14.65) | 1.36 (2.27) | 45.36 (5.29) | 5.57 (3.98) |
| Superior Temporal | 30.23 (14.53) | 1.69 (3.38) | 44.00 (5.71) | 6.57 (5.23) |
3.2 Selective Reminding Test (SRT)
The mean scores achieved by each group on the SRT are presented in Table 3. A series of one-way ANOVAs showed that the three groups differed in the total number of words recalled across the six learning trials (F(2,51)=62.65; p<.001), the total number of words recalled from long-term (LTS; F(2,51)=61.52; p<.001) or short-term (STS; F(2,51)=5.86; p<.005) storage, and the percentage of the 10 words that were recognized (F(2,50)=8.80; p<.001). Post-hoc pair-wise comparisons showed that patients with DLB and patients with AD performed worse than NC participants on all four measures (all p’s<.001). DLB and AD patients did not differ in the total number of words recalled (t(25)=0.08; p=.94), the number of words recalled from LTS (t(25)=1.41; p=.17), or recognition (t(24)=1.20; p=.24). DLB recalled fewer words from STS than did patients with AD (t(25)=2.13; p=.04).
Table 3.
Mean scores achieved by patients with Dementia with Lewy Bodies (DLB), patients with Alzheimer’s Disease (AD) and Normal Control (NC) participants on the Buschke Selective Reminding Test. Measures include the total number of words recalled across six 10-word trials (Total Recall), the number of words recalled from long-term storage (LTS), the number of words recalled from short-term storage (STS), and the number of words recognized after a 5-minute delay. (Standard deviations are shown in parentheses.)
| NC (n=25) | DLB (n=14) | AD (n=14) | |
|---|---|---|---|
| Total Recall | 42.8 (7.1) | 17.4 (9.4) | 17.7 (8.5) |
| Delayed Recognition | 100.0 (0.0) | 87.1 (13.8) | 93.3 (12.3) |
|
| |||
| Long-Term Storage | 35.9 (10.8) | 9.3 (10.0) | 5.2 (3.5) |
| Short-Term Storage | 6.9 (4.1) | 8.1 (4.2) | 12.5 (6.4) |
3.3 Recognition Span Test
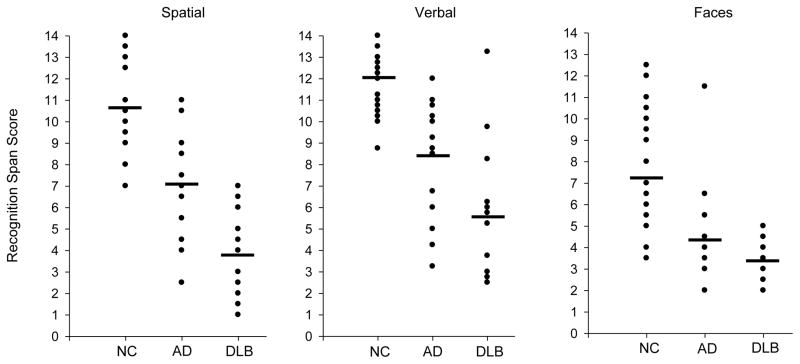
The mean recognition span scores achieved by each group in each of the three stimulus conditions are presented in Figure 1. A 3 (group) × 3 (stimulus condition) repeated measures Analysis of Variance (ANOVA) yielded significant group (F(2,50)=54.73; p<.001) and stimulus condition effects (F(1,50)=37.89; p<.001), and a significant Group × Condition interaction effect (F(2,50)=7.59; p<.001). Pair-wise comparisons using post-hoc t-tests showed that patients with DLB and patients with AD scored lower than NC participants in each of the recognition span conditions (all p’s<.004). Patients with DLB scored lower than patients with AD in the spatial span (t(26)=4.02; p<.001) and verbal span (t(26)=2.44; p=.022) conditions. The patient groups did not differ significantly in the faces span condition (t(26)=1.44; p=.16). The worse performance of patients with DLB compared to patients with AD remained evident for both the spatial span (F(1,24)=62.65; p<.001) and verbal span (F(1,24)=15.20; p<.001) conditions Analysis of Covariance (ANCOVA) was used to control for secondary memory performance measured by LTS on the SRT. The same pattern of results was also obtained when patients on psychoactive medications were excluded from the analyses.
Figure 1.
Recognition memory span scores achieved by individual patients with Dementia with Lewy Bodies (DLB), patients with Alzheimer’s Disease (AD) and Normal Control (NC) participants in the spatial, verbal and faces stimulus conditions. The mean recognition memory span score for each group in each condition is indicated by a horizontal black bar. Note that several individual points for each group in each condition overlap.
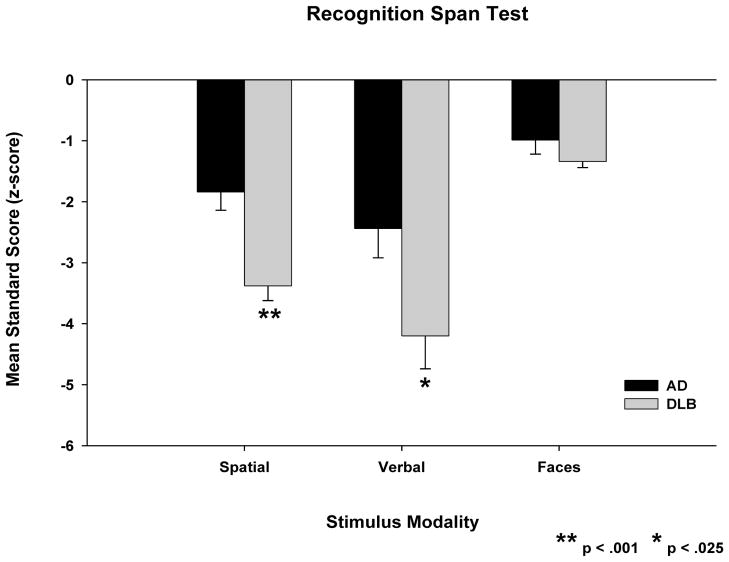
Post-hoc paired t-tests showed that NC participants scored significantly better in the verbal span condition than in the spatial (t(24)=2.53; p<.02) or faces span (t(24)=8.74; p<.001) conditions, and significantly better in the spatial span condition than in the faces span condition (t(24)=6.73; p<.001). Therefore, span scores of patients with DLB and patients with AD were converted to standard scores (z-scores based on the mean and standard deviation of the NC participants) to compare the severity of their deficits across the three recognition span conditions. The mean z-scores for the DLB and AD patient groups in each condition are shown in Figure 2. A 2 (group) × 3 (stimulus condition) repeated measures ANOVA yielded significant group (F(1,26)=10.93; p<.003) and stimulus condition (F(1,26)=55.09; p<.001) effects and a significant Group × Condition interaction effect (F(1,26)=9.27; p<.005). Post-hoc t-tests showed that DLB patients were significantly more impaired than patients with AD in the spatial span (t(26)=4.02; p<.001) and verbal span (t(26)=2.44; p<.03) conditions. The groups had similar degrees of impairment in the faces span condition (t(26)=1.44; p=.16). Post-hoc paired t-tests showed that DLB patients were significantly more impaired in the verbal span (t(13)=5.52; p<.001) and spatial span (t(13)=9.75; p<.001) conditions than in the faces span condition, and there was a trend for them to be more impaired in the verbal span condition than in the spatial span condition (t(13)=2.06; p=.06). Patients with AD were significantly more impaired in the verbal span (t(13)=3.07; p=.009) and spatial span (t(13)=2.59; p=.02) conditions than in the faces span condition. Their degrees of impairment in the verbal span and spatial span conditions were not significantly different (t(13)=1.48; p=.16).
Figure 2.
Mean recognition memory span test standard scores (i.e., z-scores) scores of patients with Dementia with Lewy Bodies (DLB) and patients with Alzheimer’s Disease (AD) in the spatial, verbal and faces stimulus conditions. Standard scores are based on the mean and standard deviation of normal control participants in each stimulus condition. Error bars are the standard error of the mean.
Receiver-operating-characteristic (ROC) curves (not shown) were plotted to compare how effectively the spatial and verbal recognition memory span tasks differentiated patients with DLB from those with AD. The area under the curve was .86 for the spatial span condition and .77 for the verbal condition. Because it was decided a priori that sensitivity and specificity were of equal importance, the optimal cut-off was chosen to be where the sum of sensitivity and specificity reached a maximum value. In the spatial condition, an optimal cut-off span of 6 or less provided 86% sensitivity for having DLB and 71% specificity for not having DLB (i.e., for having AD), for an overall accuracy of 79%. In the verbal condition, an optimal cut-off span of 6.25 or less provided 79% sensitivity for having DLB and 71% specificity for not having DLB (i.e., for having AD), for an overall accuracy of 75%.
The mean numbers of words freely recalled by each group following the verbal span test are shown in Table 4. A 3 (group) × 2 (15-sec vs. 2-min delay interval) repeated measures ANOVA yielded significant group (F(2,50)=28.70; p<.001) and delay interval (F(1,50)=23.68; p<.001) effects. The group × delay interval interaction effect was not significant (F(2,50)=0.91; p=.41). Pair-wise comparisons showed that patients with DLB and patients with AD recalled fewer words than NC participants in both of the delay interval conditions (all p’s<.001), but did not differ from each other.
Table 4.
Mean number of words recalled by patients with Dementia with Lewy Bodies (DLB), patients with Alzheimer’s Disease (AD) and Normal Control (NC) participants in the 15-second and 2-minute delayed recall conditions of the verbal Recognition Span Test. (Standard deviations are shown in parentheses.)
| NC (n=25) | DLB (n=14) | AD (n=14) | |
|---|---|---|---|
| 15-Second Delay | 7.04 (1.97) | 2.93 (3.20) | 2.64 (2.02) |
| 2-Minute Delay | 6.24 (1.83) | 2.14 (2.80) | 1.29 (1.54) |
3.4 Modified Wisconsin Card Sorting Test (mWCST)
The mean number of sorts (principles) achieved, and the mean numbers of non-perseverative and perseverative errors committed, by each group on the mWCST are presented in Table 5. A series of one-way ANOVAs showed that the three groups differed in the number of sorts achieved (F(2,51)=33.90; p<.001), the number of non-perseverative errors (F(2,51)=12.30; p<.001) and the number of perseverative errors (F(2,51)=11.03; p<.001). Post-hoc pair-wise comparisons showed that patients with DLB performed worse than NC participants on all three measures (all p’s<.001), while patients with AD performed worse than NC participants on the measures of sorts achieved and perseverative errors (all p’s<.001) but not non-perseverative errors (t(36)=1.94; p=.06). Patients with DLB achieved fewer sorts than patients with AD (t(26)=2.40; p=.02) and made more non-perseverative errors (t(26)=2.48; p=.02). The DLB and AD patients did not differ in number of perseverative errors (t(26)=0.29; p=.78).
Table 5.
Mean number of sorts (principles) achieved and mean numbers of perseverative and non-perseverative errors committed by patients with Dementia with Lewy Bodies (DLB), patients with Alzheimer’s Disease (AD) and Normal Control (NC) participants on the modified Wisconsin Card Sorting Test. (Standard deviations are shown in parentheses.)
| NC (n=25) | DLB (n=14) | AD (n=14) | |
|---|---|---|---|
| Sorts Achieved | 5.29 (1.08) | 1.36 (1.21) | 2.93 (2.13) |
| Non-Perseverative Errors | 7.79 (4.74) | 18.36 (8.28) | 11.36 (6.57) |
| Perseverative Errors | 1.67 (2.58) | 12.57 (10.35) | 11.43 (10.87) |
4. Discussion
The present study directly compared the performance of patients with AD and those with DLB on a recognition span task that requires effective interaction between working memory and secondary memory for optimal performance. Results showed that DLB patients performed substantially worse than AD patients on the recognition span task with both verbal and spatial stimuli. Both groups performed worse than the healthy control group in these stimulus modalities, but the degree of impairment was significantly greater in DLB than in AD. Moreover, a comparison across stimulus modalities indicated that while patients with AD displayed similar levels of impairment with words and spatial locations, there was a near-significant (p=.06) trend for the recognition span deficit of patients with DLB to be greater with words than with spatial locations. Recognition spans for faces did not differ in the two patient groups, and both groups were only marginally impaired relative to NC participants with this stimulus modality (i.e., average z-scores were −1 to −1.5 standard deviations below normal). This is likely due to the fact that the faces condition was the most difficult condition for NC participants and therefore less sensitive than spatial or verbal modalities to impairment in the patient groups.
In contrast to the differences observed on the recognition memory span test, DLB and AD patients in the present study exhibited similar secondary memory deficits. The patient groups did not differ in the total number of words recalled across the six learning trials of the SRT, or in the number of those words recalled from LTS (although AD patients recalled numerically fewer words from LTS than did DLB patients). Recall from LTS is a particularly salient measure of secondary memory that is not biased by the ability to use immediate memory to repeat back to-be-remembered items that were just heard (Buschke, 1973). DLB and AD patients also performed similarly on the delayed recall aspect of the recognition memory span test. There was no difference in the number of words used in the recognition memory span test that were freely recalled by DLB or AD patients after either 15-second or 2-minute delay intervals. Both patient groups recalled fewer words than NC participants at both delays.
While traditional span tasks rely primarily on working memory processes mediated by fronto-striatal circuits (Frank et al., 2001), the recognition memory span task also engages long-term memory (i.e., secondary memory) processes mediated by the diencephalic-medial temporal lobe memory system (Squire, 1992). Previous findings from neuroimaging studies in neurologically intact subjects have shown that fronto-striatal systems cooperatively interact with the medial temporal lobe system to support learning on secondary memory tasks that also place high demands on working memory processes (Sadeh et al., 2011). Because the recognition span task engages episodic memory encoding under conditions that require aspects of working memory such as the maintenance of relevant stimuli and the filtering of irrelevant stimuli, the task should elicit interaction between working memory and secondary memory systems. The task would therefore be particularly sensitive at detecting memory impairment in disorders that produce disruption to both memory systems rather than to either memory system in isolation. Thus, the greater recognition span impairment of the DLB patients is not likely to depend solely on the integrity of secondary memory processes that were comparably disrupted in both DLB and AD, but may depend as well upon working memory processes that are more severely affected in DLB than in AD (e.g., Sahgal et al., 1995; Calderon et al., 2001).
This interpretation of the greater recognition memory span deficit we observed in DLB than in AD patients is consistent with differences in the distribution of neuropathology in the two disorders. While both disorders are associated with pathology in medial temporal lobe regions that are crucial for secondary memory, DLB entails additional subcortical pathology that affects the integrity of fronto-striatal circuits that are thought to play an important role in working memory (Hansen et al., 1993; Harding and Holliday, 2001; Horimoto et al., 2003; Tsuboi and Dickson, 2005). Consistent with this additional pathology, a number of previous studies with clinically-defined patient cohorts have demonstrated a greater working memory deficit in patients with DLB than in those with AD (Sahgal et al., 1995; Calderon et al., 2001). Sahgal and colleagues (1995), for example, compared the performance of DLB and AD patients on a spatial working memory task that assessed both spatial memory and the ability to use an efficient search strategy. In this task, subjects were required to search through a number of boxes presented on a computer screen in order to locate a hidden “token”. They were not to re-examine an empty box before finding the token on the current trial (i.e., a within-search error), nor were they to search a box in which the token had been found on a previous trial (i.e., a between-search error). The results showed that patients with DLB made more within-search and between-search errors than those with probable AD, but the groups did not differ in the search strategies they used to complete the task. The possibility of greater fronto-striatal dysfunction in patients with DLB than in those with AD was supported in the present study by significantly worse performance on the modified Wisconsin Card Sorting Test in the DLB group. While both patients groups performed worse than NC participants, patients with DLB achieved fewer categories and made more non-perseverative errors than patients with AD on this independent assessment of the integrity of fronto-striatal circuits.
The comparable secondary memory deficits exhibited by DLB and AD patients in the present study is consistent with the extensive medial temporal lobe damage that occurs in the two disorders. A number of studies using neuropathologic (Lippa et al., 1994; 1998) or magnetic resonance imaging ( Hashimoto et al., 1998; Barber et al., 2000, 2001) procedures have shown atrophy of medial temporal lobe structures important for memory (e.g., hippocampus, entorhinal cortex, parahippocampal gyrus) in both disorders. Furthermore, the present study showed similar levels of amyloid plaque pathology in the hippocampus of AD and DLB patients. It should be noted, however, that patients with DLB had significantly less hippocampal tangle pathology than patients with AD and this his may account for the numerically smaller secondary memory deficit that was observed in the DLB patients compared to the AD patients.
The greater degree of impairment patients with DLB exhibited on the recognition span task with words than with spatial locations indicates that their recognition span deficit cannot be attributed solely to their previously documented visuospatial deficits (for review, see Metzler-Baddley, 2007). Instead, their performance most likely reflects deficits in memory processes that are commonly tapped by both versions of the task. Indeed, the slightly greater impairment on the word version rather than the spatial version of the task is consistent with the view that the recognition span impairment of DLB patients will increase as the need for interaction between working memory and secondary memory systems increases within the task (i.e., under episodic encoding conditions which place heavy demands on complex working memory operations). While both the word and spatial conditions place comparable demands on the maintenance of relevant information within working memory, the word version also requires the ability to filter out irrelevant information from working memory. In the spatial version of the task, blank disks are incrementally placed on different locations on the board, and the participants must simply point to the location of the most recently placed disk. In the word version of the task, in contrast, labeled disks are incrementally placed in random locations, and participants must remember the words while ignoring the potentially more salient spatial locations of the disks. The increased demand the word version of the task places on maintenance and filtering operations intensifies the need for interaction between working memory and secondary memory systems. Thus, this version of the task might be particularly sensitive to the disruption of both working memory and secondary systems that occurs in DLB.
In conclusion, the unique demands that the recognition memory span task places upon the effective interaction between working memory and secondary memory systems may make this task particularly suitable for detecting cognitive impairment in patients with DLB, and for distinguishing patients with DLB from patients with AD who present with comparable secondary memory impairments. Patients with DLB were substantially more impaired than patients with AD in their ability to effectively combine working memory and secondary memory processes in the service of successful episodic encoding on the recognition span task. The conjoint disruption of medial temporal lobe and fronto-striatial memory systems and their interaction in DLB may lead to unique recognition memory span deficits that are not seen in other patients groups. The present findings further suggest that DLB may be an ideal model system for increasing our basic understanding of the ways in which the medial temporal lobe and fronto-striatal systems interact to support learning and memory in neurologically intact populations.
Acknowledgments
We thank the participants and staff of the Shiley-Marcos Alzheimer’s Disease Research Center at the University of California, San Diego. This study was supported by NIH grants AG12963, AG05131 and NS049298, and the Shiley-Marcos Alzheimer’s Disease Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballard C, Patel A, Oyebode F, Wilcock G. Cognitive decline in patients with Alzheimer’s disease, vascular dementia and senile dementia of the Lewy body type. Age and Ageing. 1996;25:209–213. doi: 10.1093/ageing/25.3.209. [DOI] [PubMed] [Google Scholar]
- Barber R, Ballard C, McKeith IG, Gholkar A, O’Brien JT. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology. 2000;54:1304–1309. doi: 10.1212/wnl.54.6.1304. [DOI] [PubMed] [Google Scholar]
- Barber R, McKeith IG, Ballard C, Gholkar A, O’Brien JT. A comparison of medial and lateral temporal lobe atrophy in dementia with Lewy bodies and Alzheimer’s disease: magnetic resonance imaging volumetric study. Dementia and Geriatric Cognitive Disorders. 2001;12:198–205. doi: 10.1159/000051258. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12:543–550. [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Calderon J, Perry RJ, Erzinclioglu SW, Berrios GE, Dening TR, et al. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. Journal of Neurology Neurosurgery and Psychiatry. 2001;70:157–164. doi: 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor DJ, Salmon DP, Sandy TJ, Galasko D, Hansen LA, Thal L. Cognitive profiles of autopsy-confirmed Lewy body variant vs. pure Alzheimer’s disease. Archives of Neurology. 1998;55:994–1000. doi: 10.1001/archneur.55.7.994. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cognitive, Affective and Behavioral Neuroscience. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Hamilton JM, Salmon DP, Galasko D, Delis DC, Hansen LA, Masliah E, et al. A comparison of episodic memory deficits in neuropathologically-confirmed Dementia with Lewy bodies and Alzheimer’s disease. Journal of the International Neuropsychological Society. 2004;10:689–697. doi: 10.1017/S1355617704105043. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Masliah E, Galasko D, Terry RD. Plaque-only Alzheimer disease is usually the Lewy body variant, and vice versa. Journal of Neuropathology and Experimental Neurology. 1993;52:648–654. doi: 10.1097/00005072-199311000-00012. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Masliah E, Quijada-Fawcett S, Rexin D. Entorhinal neurofibrillary tangles in Alzheimer disease with Lewy bodies. Neuroscience Letters. 1991;129:269–272. doi: 10.1016/0304-3940(91)90478-c. [DOI] [PubMed] [Google Scholar]
- Hansen L, Salmon D, Galasko DR, Masliah E, Katzman R, DeTeresa R, et al. The Lewy body variant of Alzheimer’s disease: A clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- Hansen L, Samuel W. Criteria for Alzheimer’s disease and the nosology of Dementia with Lewy bodies. Neurology. 1997;48:126–132. doi: 10.1212/wnl.48.1.126. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathologica. 2001;102:355–363. doi: 10.1007/s004010100390. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Kitagaki H, Imamura T, Hirono N, Shimomura T, Kazui H, et al. Medial temporal and whole-brain atrophy in dementia with Lewy bodies. Neurology. 1998;51:357–362. doi: 10.1212/wnl.51.2.357. [DOI] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum GG, Gearing M, Mirra SS, Welsh-Bohmer KA, Peterson B, et al. Comparison of Lewy body variant of Alzheimer’s disease with pure Alzheimer’s disease: Consortium to establish a registry for Alzheimer’s disease, part XIX. Neurology. 1999;52:1839–1844. doi: 10.1212/wnl.52.9.1839. [DOI] [PubMed] [Google Scholar]
- Horimoto Y, Matsumoto M, Nakazawa H, Yuasa H, Morishita M, Akatsu H, et al. Cognitive conditions of pathologically confirmed dementia with Lewy bodies and Parkinson’s disease with dementia. Journal of Neurological Science. 2003;216:105–108. doi: 10.1016/s0022-510x(03)00220-x. [DOI] [PubMed] [Google Scholar]
- Levy DA, Manns JR, Hopkins RO, Gold JJ, Squire LR. Impaired visual and odor recognition memory span in patients with hippocampal lesions. Learning & Memory. 2003;10:531–536. doi: 10.1101/lm.66703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa CF, Johnson R, Smith TW. The medial temporal lobe in dementia with Lewy bodies: a comparative study with Alzheimer’s disease. Annals of Neurology. 1998;43:102–106. doi: 10.1002/ana.410430117. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Smith TW, Swearer JM. Alzheimer’s disease and Lewy body disease: a comparative clinicopathological study. Annals of Neurology. 1994;35:81–88. doi: 10.1002/ana.410350113. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer’s disease and Parkinson’s disease with dementia. Cortex. 2007;43:583–600. doi: 10.1016/s0010-9452(08)70489-1. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Moss MB, Albert MS, Butters N, Payne M. Differential patterns of memory loss among patients with Alzheimer’s disease, Huntington’s disease, and alcoholic Korsakoff’s syndrome. Archives of Neurology. 1986;43:239–246. doi: 10.1001/archneur.1986.00520030031008. [DOI] [PubMed] [Google Scholar]
- Nelson HE. A modified sorting test sensitive to frontal lobe deficits. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- NIA and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiology of Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- Rub U, Del Tredici K, Schult C, Ghebremedhin E, de Vos RA, Jansen SE, et al. Parkinson’s disease: the thalamic components of the limbic loop are severely impaired by alpha-synuclein immunopositive inclusion body pathology. Neurobiology of Aging. 2002;23:245–254. doi: 10.1016/s0197-4580(01)00269-x. [DOI] [PubMed] [Google Scholar]
- Sadeh T, Shohamy D, Levy DR, Reggev N, Maril A. Cooperation between the hippocampus and the striatum during episodic encoding. Journal of Cognitive Neuroscience. 2011;23:1597–1608. doi: 10.1162/jocn.2010.21549. [DOI] [PubMed] [Google Scholar]
- Sahgal A, McKeith IG, Galloway PH, Tasker N, Steckler T. Do differences in visuospatial ability between senile dementias of the Alzheimer and Lewy body types reflect differences solely in mnemonic function? Journal of Clinical and Experimental Neuropsychology. 1995;17:35–43. doi: 10.1080/13803399508406579. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annual Review of Psychology. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Galasko D, Hansen LA, Masliah E, Butters N, Thal LJ, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain and Cognition. 1996;31:148–165. doi: 10.1006/brcg.1996.0039. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Granholm E, McCullough D, Butters N, Grant I. Recognition memory span in mildly and moderately demented patients with Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 1989;11:429–443. doi: 10.1080/01688638908400904. [DOI] [PubMed] [Google Scholar]
- Scimeca JM, Badre D. Striatal contributions to declarative memory retrieval. Neuron. 2012;75:380–392. doi: 10.1016/j.neuron.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura T, Mori E, Yamashita H, Imamura T, Hirono N, Hashimoto M, et al. Cognitive loss in dementia with Lewy bodies and Alzheimer disease. Archives of Neurology. 1998;55:1547–1552. doi: 10.1001/archneur.55.12.1547. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Annals of Neurology. 1981;10:184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Dickson DW. Dementia with Lewy bodies and Parkinson’s disease with dementia: are they different? Parkinson’s Disease and Related Disorders. 2005;11:S47–51. doi: 10.1016/j.parkreldis.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Walker Z, Allen R, Shergill S, Katona C. Neuropsychological performance in Lewy body dementia and Alzheimer’s disease. British Journal of Psychiatry. 1997;170:156–158. doi: 10.1192/bjp.170.2.156. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]