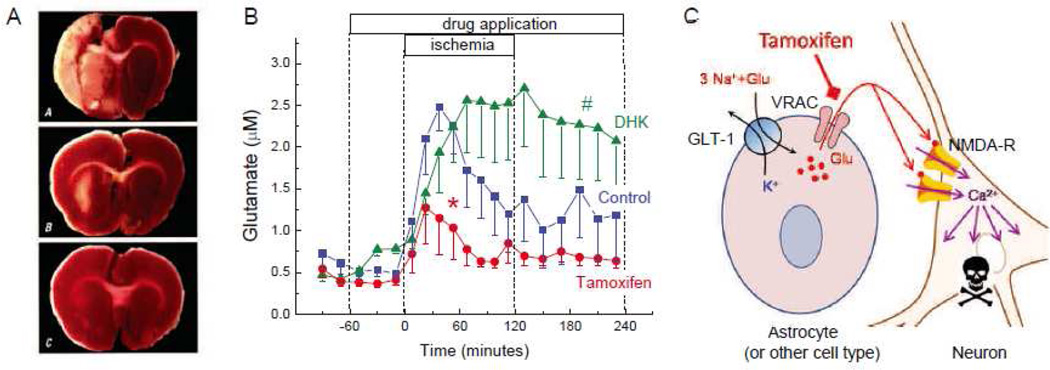
Fig. 3. The VRAC inhibitor tamoxifen potently protects rat brain against damage in experimental stroke model, and reduces the intraischemic glutamate release in rat cortical tissue.
A, Representative images of brain sections from rats subjected to 2-h experimental stroke and evaluated 3 days after ischemia. Animals were treated with vehicle (A), or the VRAC blocker tamoxifen (5 mg/kg) given i.v. either 10 min prior (B), or 3 h after initiation of stroke (C). At the end of experiment rats were euthanized and their brains were sliced and stained with triphenyltetrazolium chloride to visualize tissue damage (infarcted tissue is unstained). Reproduced with permission from H.K. Kimelberg et al. (2000). B, Dynamics of the microdialysate glutamate levels during and after focal cerebral ischemia. Vehicle control (5% DMSO), the VRAC blocker tamoxifen (50 µM), or the inhibitor of the glial glutamate transporter GLT-1 dihydrokainate (DHK, 1 mM) were delivered through the microdialysis probe placed in the ischemic penumbra. *p<0.05, tamoxifen vs. DMSO and DHK during ischemia; #p<0.05, DHK vs tamoxifen and control after ischemia (repeated measures ANOVA). Modified with permission from P.J. Feustel et al. (2004). C, Hypothetical representation of the processes in the ischemic penumbra, based on the results of microdialysis experiments. Pathological swelling of astrocytes (and perhaps other cells) triggers glutamate release via the VRAC. Increased glutamate levels cause Ca2+-dependent damage and death of neuronal cells due to excessive activation of neuronal NMDA receptors. In penumbra, glial transporter GLT-1 continues to take up extracellular glutamate and, therefore, addition of DHK leads to high sustained glutamate levels during and after ischemia.