Abstract
Many therapeutically relevant natural products are biosynthesized by the action of giant mega-enzyme assembly lines. By leveraging the specificity, promiscuity, and modularity of assembly lines, a variety of strategies have been developed that enable the biosynthesis of modified natural products. This review briefly summarizes recent structural advances related to natural product assembly lines, discusses chemical approaches to probing assembly line structures in the absence of traditional biophysical data, and surveys efforts that harness the inherent or engineered promiscuity of assembly lines for the synthesis of non-natural polyketides and nonribosomal peptide analogues.
Keywords: Combinatorial biosynthesis, synthetic biology, enzyme engineering, polyketide synthases, non-ribosomal biosynthesis
INTRODUCTION
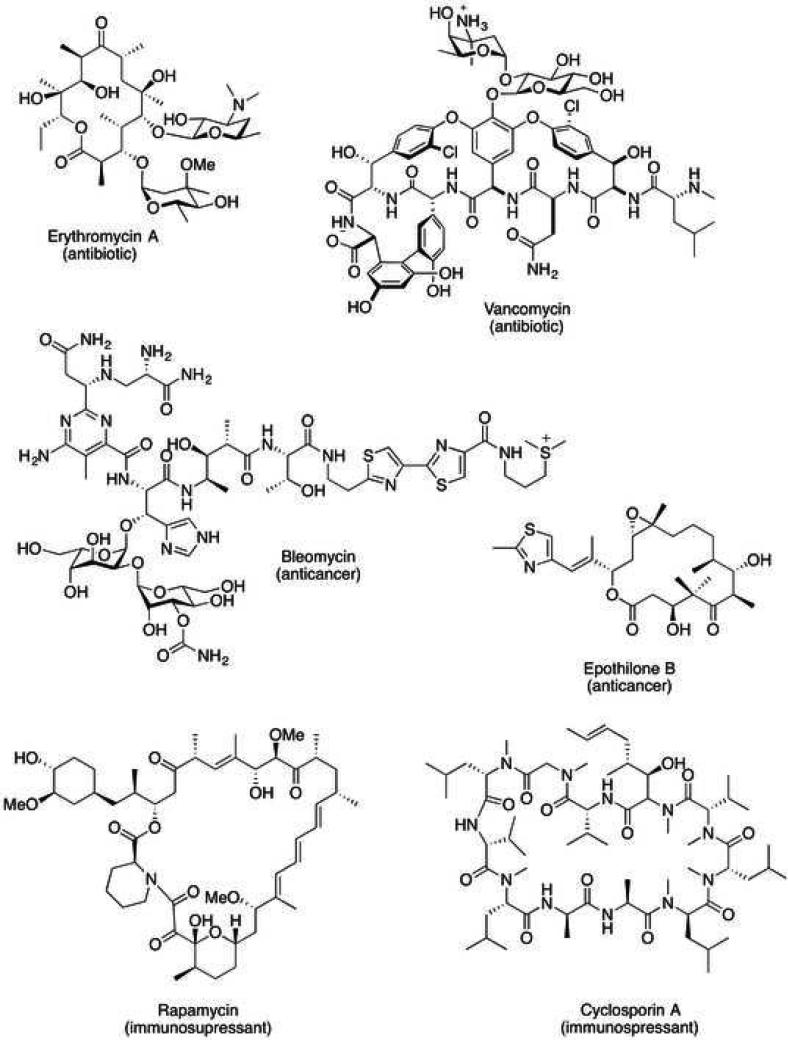
Therapeutic polyketide and non-ribosomal peptide natural products have been invaluable for the treatment of a variety of diseases including bacterial infections (e.g. erythromycin and vancomycin), cancer (e.g. epothilione and bleomycin), and immunosuppression (e.g. rapamycin and cyclosporin) (Figure 1). Notably, approximately 65% of the current drugs on the market are derived or inspired from natural products [98,97]. Consequently, polyketides and non-ribosomal peptides, including their biologically active precursors and derivatives, have proven popular targets for total synthesis [144,76]. Though there has been much success in this area, most synthetic routes to complex polyketides and non-ribosomal peptides require dozens of chemical steps and are generally low yielding [62]. Furthermore, this approach is often not amenable to facile analoging. In contrast, Nature has arrived at a remarkably efficient strategy for producing complex structures by assembling small molecule building blocks via enzymatic assembly lines.
Figure 1.
Examples of clinically used natural products biosynthesized via assembly lines.
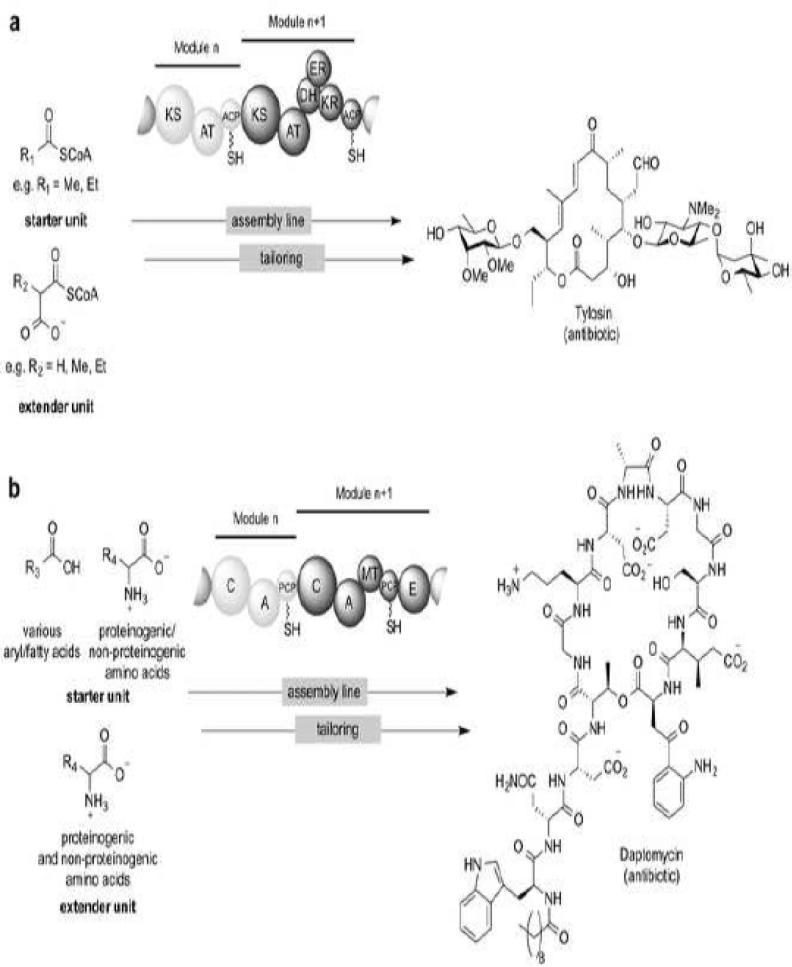
The biosynthesis of many clinically relevant polyketides is coordinated by enzymatic assembly lines called type I polyketide synthases (PKSs) via the selection and installation of acyl-CoA derived building blocks called starter units and extender units [114]. Type I PKSs are organized in a modular fashion, whereby a module of enzyme activities is responsible for building block selection and installation into the polyketide (Figure 2a). Type I PKSs are composed minimally of three domains: acyl carrier protein (ACP), acyltransferase (AT), and ketosynthase (KS). The ACP is post-translationally modified with a phosphopantetheine arm, which serves to shuttle substrates and intermediates through the assembly line. Additionally, PKSs can contain optional domains such as the ketoreductase (KR), enoylreductase (ER), dehydratase (DH), and thioesterase (TE). Fungal PKSs are a variant of type I PKSs in which a given module acts in an iterative fashion [26]. In contrast to assembly line PKSs, type II PKSs possess discrete monofunctional domains [53]. Further, type III PKSs act as simple homodimers producing small aromatic compounds [1]. In a similar assembly line fashion to type I PKSs, many medicinally relevant non-ribosomal peptides are constructed from a variety of amino acids by non-ribosomal peptide synthases (NRPSs) (Figure 2b). Three primary domains are also present: the adenylation (A), peptidyl carrier protein (PCP), and condensation (C) domains as well as a variety of additional tailoring domains (e.g. cyclization, oxidation, reduction, methylation, epimerization) [131].
Figure 2.
Modular organization of natural product assembly lines. (a) Modular type I PKS organization. Module ‘n’ shows the minimal domains that are required by most PKS modules. Module ‘n+1’ includes a full complement of reductive domains. Typical starter and extender units are shown, along with an example of a polyketide produced by a type I PKS. (b) Modular NRPS organization. Module ‘n’ shows the minimal domains that are required by each NRPS module. Module ‘n+1’ includes additional domains that are often included. Typical starter and extender units are shown, along with an example of a nonribosomal peptide natural product.
Additional structural diversity is afforded by mixing PKSs and NRPSs to form hybrid assembly lines such as the zwittermicin synthase [36,10]. Polyketides and non-ribosomal peptides can be further modified by a variety of post-assembly line tailoring enzymes such as P450s [104], glycosyltransferases [137], halogenases [14], and methyltransferases [115]. Accordingly, there is an abundance of natural product structural diversity afforded by assembly lines and associated tailoring machinery. Nevertheless, natural products often require further chemical modification in order to improve biological activity and to modulate pharmacological properties. In an effort to diversify the products of polyketide and non-ribosomal peptide assembly lines, a variety of approaches involving metabolic engineering, protein engineering, and combinatorial biosynthesis have been explored. Notable examples aside, the ability to leverage the potential modularity of these systems by domain and module swapping to produce ‘non-natural’ natural products has been met with limited success. Often, chimeric assembly lines produce the desired analogue in much lower titers than the wild-type machinery [39,109,89,50]. More recently, new strategies in synthetic biology and enzyme engineering are being developed to address limitations to the scope and utility of chimeric assembly lines. Here, we review recent structural advances related to PKS and NRPS assembly lines that highlight the difficulties associated with ‘traditional’ chimera design and combinatorial biosynthesis. Then, we review current engineering efforts that leverage promiscuity of individual assembly line components and discuss future perspectives related to this area. For brevity, we will focus on engineering the assembly lines themselves and not discuss aspects that involve post-assembly tailoring. Natural product diversification strategies that utilize post-PKS/NRPS tailoring functions have been superbly reviewed elsewhere [102,78,40,115].
STRUCTURAL AND MECHANISTIC STUDIES: IMPLICATIONS FOR ASSEMBLY LINE SYNTHETIC BIOLOGY
Domain structures
Probing and elucidating the molecular architecture of natural product assembly lines is crucial to understanding the potential modularity of individual domains and modules from PKSs and NRPSs. The bulk of early structural information has been elucidated from excised domains, module fragments, or discrete trans-acting enzymes. Crystal structures are available for a variety of PKS domains [66], including ketosynthases [41], trans-acting acyltransferases [140], KS-AT didomains [120,121], ketoreductases [41], an enoyl-isomerase [42], ACPs [27], and linker domains [16]. These structures have provided insight into the mechanism and specificity of individual biosynthetic steps. For example, the crystal structure of a ketoreductase from the erythromycin PKS was used to understand and predict the stereochemical outcome of the keto-reduction [5]. Important residues were identified that could flip the stereochemical reduction of small thioesters. Unfortunately, the change in stereochemistry caused shunting or complete inactivation of chain elongation [77]. In a similar fashion, NRPS domains have also been well characterized [84,122,69,65]. The A domain has been the most studied, with the full catalytic cycle mapped out [152]. While the structure elucidation of individual domains has helped understand individual steps [9], rational reprogramming of the substrate specificity of individual domains has still been somewhat limited.
Assembly line architecture
The sheer sizes of PKS and NRPS assembly lines and requisite dynamics have not permitted the wide availability of complete module crystal structures. To date, the only crystal structure of a complete PKS/NRPS natural product assembly line module is that of the 144 kDa NRPS SrfA-C, which encodes the terminal module of surfactin biosynthesis in Bacillus subtilis [122]. More recently, several alternative biophysical techniques have emerged as powerful tools to determine the structure and dynamics of assembly lines. Most notably, Skiniotis, Smith, Sherman and coworkers described the use of single-particle cryo-electron microscopy (cryoEM) to infer the structure of the 330 kDa PKS module PikAIII from the pikromycin PKS assembly line [135,33]. This breathtaking achievement afforded several surprises and notable features with significant implications for assembly line synthetic biology [134]. Firstly, the PikAIII subunits were arranged as a symmetrical dimer as anticipated [113], but unlike previous models [154] the dimer formed a closed arch that resulted in a single reaction chamber. Secondly, the mode of interaction between a PikAIII chimera designed to mimic in trans chain transfer from the preceding module was different from that predicted by earlier analyses [64]. Thirdly, various observations suggested that the ACP phosphopantetheine and tethered intermediate make significant contributions to docking of the ACP during each step of reaction cycle. Finally, at least seven unique functional states were observed, each involving distinct interfaces with other PikAIII domains. Presently however, it is unclear whether the PikAIII structure should be adopted as a general model for modular PKSs given a number of discrepancies compared to earlier biochemical studies, including the recently reported low-resolution small-angle X-ray scattering (SAXS) structure of the 6-deoxyerythronolide B synthase (DEBS) module 3-TE [35]. For example, the arch-shaped arrangement of PikAIII orientates the active site of each domain towards the inside of the chamber. In this way, the configuration of the KS and AT are different from that observed in the DEBS KS-AT didomain crystal structures [120,121] and the SAXS DEBS module-3-TE structure. Additionally, how fewer or additional reductive domains might impact this architecture is not known. Moreover, the PikAIII structure implicates a hitherto unidentified active site entrance to the KS and is postulated to recruit the methylmalonyl-ACP extension unit. However, it is not obvious how insertion of the extender unit via this ‘bottom’ entrance could lead to decarboxylation, given the associated machinery appears to bypassed via this route. Regardless, cumulatively this new structural insight reveals a remarkably complex model of assembly line catalysis in type I PKSs. Future studies will establish whether this collective insight will be sufficient to improve the efficiency and outcome of combinatorial biosynthesis approaches that recombine different PKS components. Perhaps overcoming the incredible complexity of these assembly lines will require evolutionary approaches that rely on testing the function of large PKS libraries for various improved activities.
Chemical tools for probing protein-protein interactions
The complex protein-protein interactions orchestrated by natural product assembly lines remain poorly understood, even in light of recent structural advances that capture intact modules. In an effort to understand these often weak and transient interactions in the absence of traditional biophysical data, a variety of techniques, including the design of chemical probes have been developed.
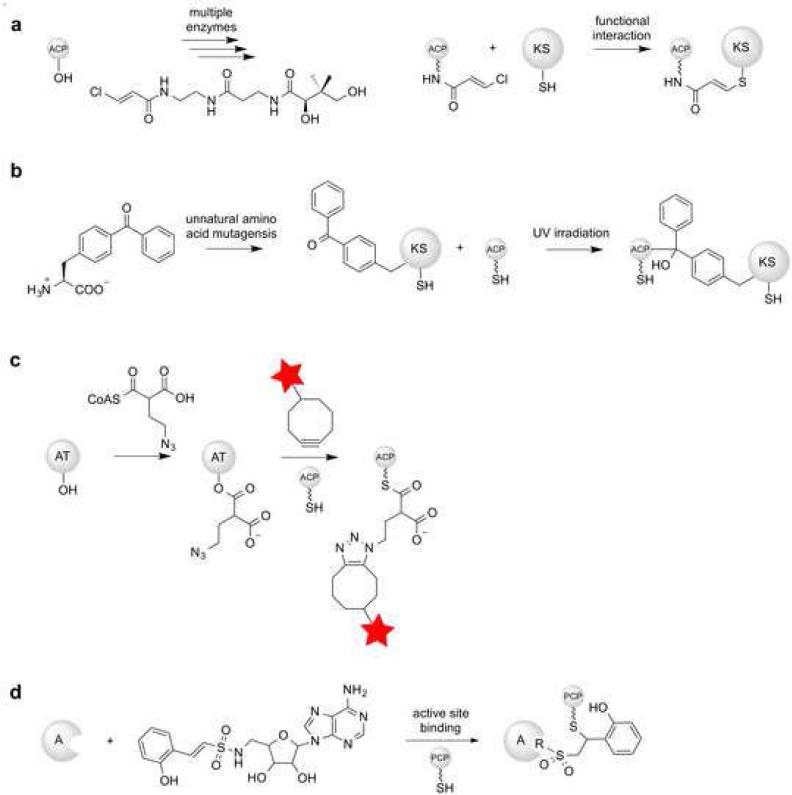
One of the most widely used approaches for understanding intramodular (within the same module) interactions is structure-based dissociation [21]. With this strategy, individual domains are excised at the genetic level, purified, and reconstituted in vitro. This technique is ideal for investigating mutational alterations and domain swapping interactions. This strategy almost always involves the carrier protein, since it is the centerpiece of every intramodular interaction. In an effort to understand non-cognate interactions, chimeric ACPs have been generated and tested in vitro [63]. Mechanism-based crosslinkers have proven popular tools to probe various intramodular interactions between the ACP/PCP and other components including domains from NRPSs, fatty acid synthases (FASs) and fungal non-reducing PKSs [58,87]. For example, unnatural phosphopantetheine analogues modified with chemical handles for crosslinking have been successfully used to investigate ACP protein-protein interactions to better understand the protein interactions involved with chain elongation (Figure 3a) [141,143]. The efficiency of mechanism-based crosslinking to the KS usually correlates with the strength of the ACP:KS interaction [142]. Moreover, this approach has been used to identify key interactions and set the stage for investigating the utility of such interfaces for combinatorial biosynthesis. More recently, similar mechanism-based crosslinking approaches have been used to provide the crystal structure of crosslinked AcpP and dehydratase from fatty acid biosynthesis [99,59]. This work provided a dynamic view of the FAS carrier protein in action during fatty acid dehydration.
Figure 3.
Chemical tools for probing and manipulating protein-protein interactions in assembly lines. (a) Enzymatic installation of a reactive unnatural phosphopantetheine analogue onto an ACP, which covalently crosslinks with the KS active site cysteine. (b) Incorporation of benzophenone group into a KS via unnatural amino acid mutagenesis. If the ACP and KS are able to interact, crosslinking takes place after UV irritation. (c) Acyltransferase-catalyzed acylation of an ACP with a reactive extender unit and subsequent cycloaddition with a strained cyclooctyne fluorophore. (d) Mechanism-based non-covalent crosslinking of an A-domain-PCP via the reactive inhibitor vinyl sulfonamide adenylate.
Other strategies for probing protein interactions in assembly lines have also leveraged chemical principles. For example, benzophenone has been site-specifically installed into carrier proteins via amber suppression technology and has been used to probe KS-ACP interactions by photocrosslinking and quantification of the crosslinked species (Figure 3b) [149]. In combination with surface scanning mutagenesis, this strategy has been used to map an ACP:KS binding interface [151]. Additionally, unnatural extender units modified with ‘click chemistry’ handles have been used to probe protein-protein interactions. For example, using an azidoethylmalonyl-CoA extender unit in combination with alanine scanning mutagenesis and chimeragenesis, Williams and colleagues mapped the interface between the promiscuous trans-AT KirCII and its cognate ACP from the kirromycin PKS assembly line (Figure 3c) [150]. Remarkably, the information acquired from this strategy allowed for a non-cognate ACP from the kirromycin assembly line to be converted into a good substrate for KirCII via a single rational amino acid substitution.
Increasingly, the structural analysis of NRPSs has also benefited from chemical approaches. For example, vinyl sulfonamide adenylate analogues have been employed to lock the catalytic conformation of A-PCP domains in order to access their crystallographic structures (Figure 3d) [118]. The structural insight from this approach proved sufficient to identify mutations that improved activity with non-native partner proteins, although it remains to be seen whether these function in vivo or if this approach could enable a new generation of analogues. In a notable advance, Schmeing and coworkers recently developed a strategy based on an engineered disulfide cross-link to isolate the thio-esterification complex of a PCP domain bound to its cognate A domain [123]. In this way, the covalent link between the thiol-aminoacyl-PCP and A domain physically locks the two domains together, limiting conformational flexibility. The short time required for this crosslinking process and the ease of installation might be advantageous in applying this strategy to a broad range of target NRPS didomains or perhaps even complete modules.
Efforts aimed at understanding and manipulating inter-modular protein interactions have focused on docking domains, which are responsible for proper docking and inter-polypeptide chain transfer during catalysis by PKS and NRPS assembly lines. Early attempts at leveraging PKS docking domains for combinatorial biosynthesis yielded mixed results, even between modules of the same system [147,92]. In some cases, the use of matched docking domains can support transfer of chain elongation intermediates between modules that do not natively associate [88,89]. Recently, a new type of inter-module linker was discovered from cyanobacteria [136]. Remarkably, these class 2 docking domains were shown to support robust transfer of chain elongation intermediates between heterologous modules. Matched docking domains have also enabled dissection of multimodular PKS polypeptides into functional monomodules, albeit with decreased titers of the final polyketide product [147]. Docking domains have also been replaced with shorter intraprotein linkers in efforts to produce functional fusion proteins, with mixed results [111,107]. In NRPSs the linkers are referred to as communication mediation domains (COM). These domains have been much more malleable than PKS linkers and have proven to be highly interchangeable [47,24,48]. Chemical crosslinkers have also been used to study NRPS module-module interactions. For example, azide and alkyne functionalized probes were used to assay NRPS COM domains via “click” reactions [57]. Structure-based dissociation can also be used to investigate inter-modular protein interactions. Trifluoromethylation of CoASH led to the installation of the phosphopantetheine onto ACP2 of DEBS to afford a holo-analogue of ACP2 [20]. This blocked version was then used in combination with acylated [KS3][AT3] from DEBS to probe the interaction with ACP2 by NMR. Further understanding of the ACP-KS:AT interaction allowed assembly line reprogramming to include an iterative chain elongation [64]. Overall, fully understanding the molecular basis of module-module interactions remains elusive and poses significant challenge for rationally reprogramming modular assembly lines.
PROMISCUITY AND ENGINEERING OF PKS/NRPS ASSEMBLY LINES
Overview of approaches
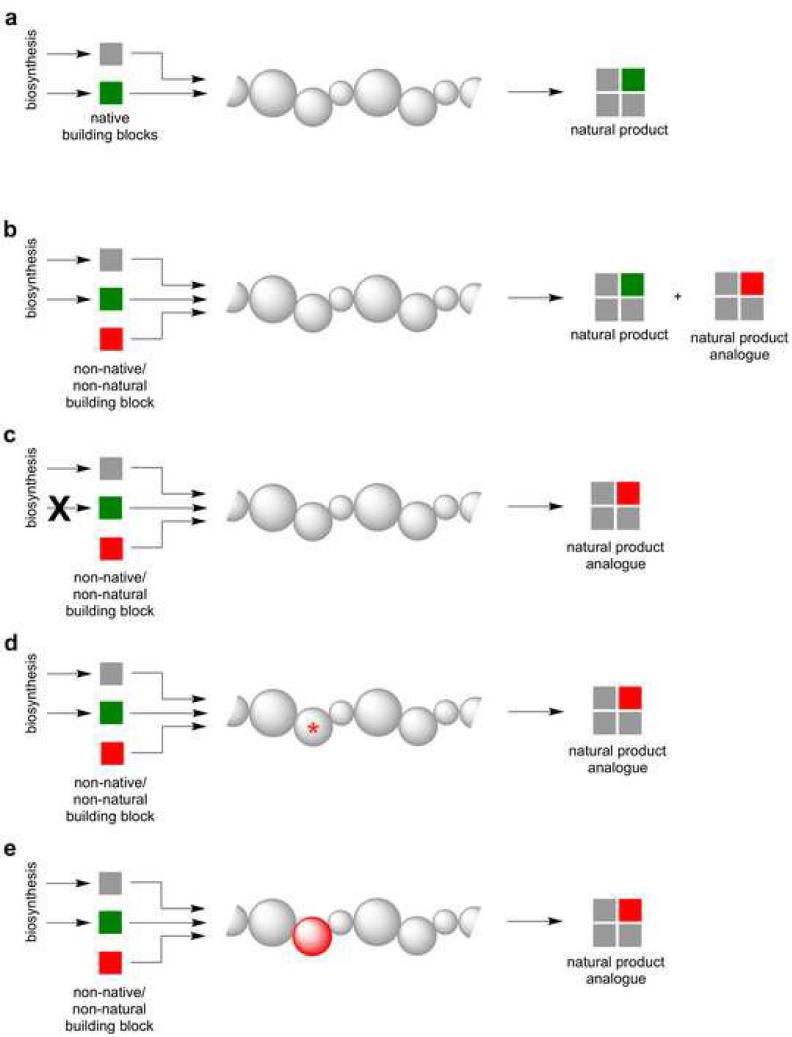
The large nature of type I PKSs and NRPSs, poor understanding of protein dynamics, and complex protein-protein interactions have hindered attempts to reprogram natural product assembly lines by domain and module swapping. Consequently, a degree of ingenuity and creativity has been required to generate new polyketide and non-ribosomal peptide analogues. Cumulatively, a variety of strategies including precursor-directed, mutasynthesis, combinatorial biosynthesis or some combination thereof have been explored (Figure 4). Rather than designing chimeric assembly lines for the production of ‘non-natural’ natural products, notable emerging successes in this area have leveraged the promiscuity of assembly line components either directly or as a platform for enzyme redesign, and this will be the focus of this section. In terms of assembly line enzymology, diversification strategies typically focus on three aspects: selection and installation of new starter and extender units, assembly line tailoring, and macrocyclization.
Figure 4.
Strategies for the production of natural product analogues that leverage the modularity of assembly lines. (a) Natural biosynthesis via a generalized assembly line. (b) Precursor-directed biosynthesis: non-native or non-natural building blocks are installed through inherent promiscuity of a module and/or domain and usually results in a mixture of the natural product and non-natural analogue. (c) Precursor-directed mutasynthesis: natural building block biosynthesis is knocked out and supplemented by a non-natural building block. (d) Enzyme-directed mutasynthesis: site-specific mutagenesis affords substrate flexibility/orthogonality and results in the regioselective incorporation of non-natural building blocks. (e) Domain swapping: substitution of the natural domain with another domain from a different assembly line allows for non-native building block incorporation.
Starter and extender units
PKS loading domains have been a popular target for diversification [93], presumably because there is a single module responsible for installation of the activated monocarboxylic acid that is inherently orthogonal to the extension modules. In addition, several loading modules exhibit relaxed specificity for the loading unit, a feature that can be easily leveraged by precursor-directed biosynthesis and mutasynthesis. For example, the natural function of the avermectin loading domain AT is to install 2-methylbutyryl-CoA or isobutyryl-CoA to provide the A and B series of avermectins, respectively. Remarkably, this loading domain is sufficiently promiscuous to furnish over 40 avermectin derivatives via feeding the corresponding carboxylic acids to a mutant producing strain [34]. Moreover, substitution of the erythromycin PKS loading domain with that of avermectin leads to generation of the expected analogues [52]. Several other loading domains have more recently shown similar substrate promiscuity [94,56,44]. A KS1 null strain of DEBS has been used to diversify starter units in addition to modifying other positions in erythromycin [60]. This mutasynthesis approach has afforded analogues modified with various non-natural alkyl substituents, in addition to fluoro, azido, and alkynl derivatives [4,43]. More recently, this approach was applied in E. coli and coupled with a colony bioassay and resulted in the identification of 15-propargyl erythromycin A [51]. This analogue is equipotent as erythromycin A and could be rapidly diversifying via ‘click chemistry’. Intriguingly, molecular docking studies suggest that the terminal acetylene binds in a different orientation to the ribosome exit tunnel, as compared to erythromycin A. In addition, a single amino acid mutation in the avermectin loading domain was sufficient to shift the specificity towards non-native acyl-SNAC substrates, at least as judged by enzymatic hydrolysis of the thioester [132]. However, the loss of catalytic efficiency towards the native substrate was largely responsible for this specificity change, and it remains to be seen whether genuine improvements in activity towards non-native or non-natural starter units can be achieved by single amino acid mutations.
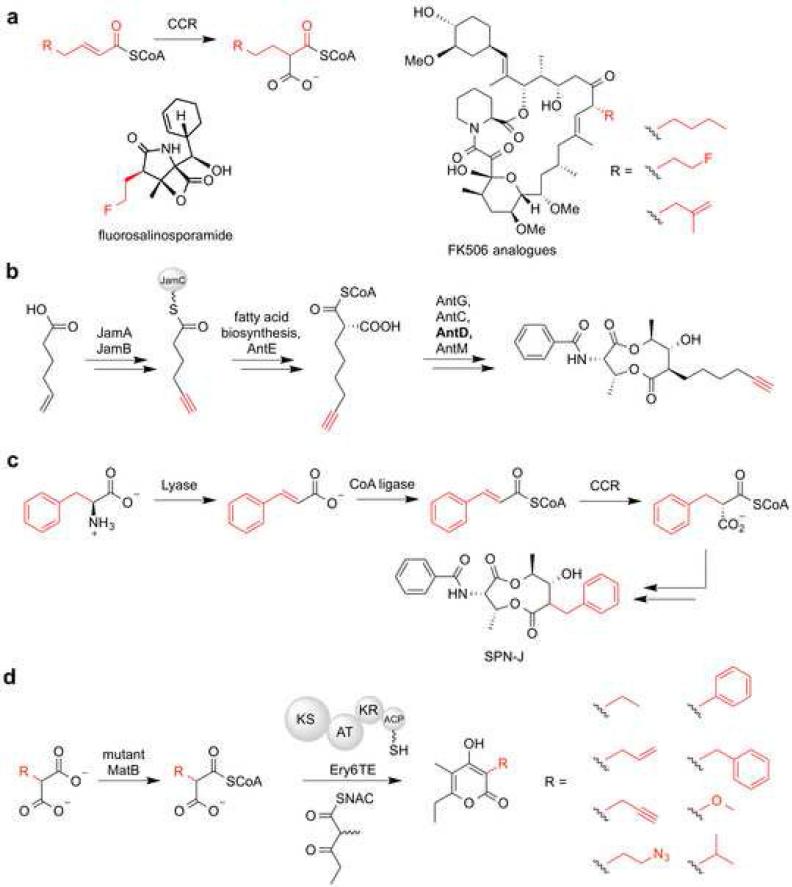
Early efforts to incorporate different extender units into polyketides focused on domain swapping. Of these examples, most were only able to swap the two most common extender units, malonyl-CoA and methylmalonyl-CoA. Besides the limited extender unit functionality, titers of the final products were severely reduced [32]. One recent notable example of effective AT-swapping produced 24-desmethylrifamycin, which showed excellent activity against rifamycin resistant strains of M. tuberculosis in good yield [101]. Though most polyketides are constructed from malonyl-CoA and methylmalonyl-CoA, a modest variety of chemical functionality is also available, including extender units modified at the C2 position with various alkyl chains, amines, hydroxyls, and halogens [138,18]. Many of the more unusual extender units are biosynthesized using crotonyl-CoA reductase (CCR) or ACP-linked pathways (Figure 5a). Some of these extender units require multistep enzymatic reactions that are not easy to manipulate. Nevertheless, several CCRs have shown to be quite flexible towards alternative unsaturated carboxylic acid precursors. Moreover, the associated ATs also seem to be promiscuous and this has led to the generation of polyketide analogues with modest structural changes (Figure 5a) [37]. Notably, some atypical extender units are generated by dedicated modules that resemble type I/type II PKSs. By knocking out part of such a pathway for allymalonyl-CoA biosynthesis in the FK506 producer and by feeding in a series of carboxylic acids, several novel FK506 analogues were generated [91]. Remarkably, by combining enzymatic machinery from the FK506 producer allylmalonyl-CoA pathway with that from the isobutyrylmalonyl-CoA pathway of the ansalactam producer, a hybrid pathway for isobutenylmlonyl-CoA was created to afford the corresponding 36-methyl-FK506 analogue (Figure 5a) [79]. Recently, a de novo pathway for extender units functionalized with terminal alkynes and alkenes was described and was utilized to generate analogues of antimycin (Figure 5b) [156], the biosynthesis of which involves a single PKS extension module. Coupling this novel extender generation system to other PKSs will likely require engineering the specificity of target ATs. Interestingly, a biosynthetic pathway for aromatic extender unit acyl-CoAs from amino acids was recently elucidated and used to target a promiscuous AT domain in the splenocin PKS (Figure 5c) [19].
Figure 5.
Extender unit generation for polyketide engineering. (a) Production of unnatural malonyl-CoA extender units via crotonyl-CoA reductase and subsequent installation into salinosporamide and FK506. (b) Installation of an alkyne into antimycin via a novel pathway (c) Generation and installation of aryl-CoA analogues into splenocin. (d) MatB-generated library of diverse malonyl-CoA extender units.
To complement lengthy, complex, and specialized extender unit pathways, chemically synthesized N-acetylcysteamine (SNAC) analogues or biosynthesized malonyl-CoA analogues offer the ability to provide highly diverse extender units. Similarly to specialized biosynthetic pathways, these strategies also offer the potential to generate polyketide analogues in situ that are modified with “click” handles. For example, a variety of extender units have been provided as the SNAC thioester and installed into several polyketide natural products by feeding these cell permeable substrates into producing organisms and utilizing inherent extender unit promiscuity of PKSs via precursor directed biosynthesis [12,117,148]. Alternatively, structure-guided mutagenesis, driven by the recently described MatB crystal structure [55], has afforded malonyl-CoA synthetase mutants with broad specificity and provide a facile route to non-natural and non-native extender units, including those modified with such chemical handles for ‘click chemistry’ (Figure 5d) [71,73]. Interestingly, the promiscuity of the terminal extension module of DEBS was sufficient to utilize a total of nine non-native and non-natural extender units provided by an engineered synthetase [72]. Notably, wild-type malonyl-CoA synthetase was demonstrated to provide the non-natural extender unit fluoromalonyl-CoA, albeit poorly [130]. Subsequently, the halogenated building block was incorporated into a simple diketide by NphT7 and reduced by PhaB. Moreover, fluoromalonyl-CoA was utilized by the terminal extension module of DEBS, and by a bimodular DEBS system when complemented with a malonyl-CoA specific trans-AT domain to afford triketide pyrones and tetraketide lactones, respectively [130]. Other approaches that rely on trans-ATs are limited by the inherent stringent specificity towards malonyl-CoA. The remarkable extender unit promiscuity of the kirromycin trans-AT KirCII [71] might be leveraged to generate a wider range of analogues.
Combinatorial biosynthesis strategies have been quite successful using NRPSs [109,108], especially with cyclic lipopeptide antibiotic pathways such as daptomycin and A54145. A combination of gene deletions and module swapping was successful in generating over 40 lipopeptides, some of which displayed improved resistance to inhibition by bovine surfactant [6,100]. In particular, deletion of genes involved in the biosynthesis of non-proteinogenic amino acids has proven effective at generating lipopeptide analogues in good yields [2]. Nevertheless, precursor-directed approaches have also been used to leverage the building bock promiscuity of NRPSs. For example, bicyclic amino acids were fed into the sansamycin Streptomyces producing strain, affording a unique analog with a 8-fold lower MIC against M. tuberculosis [145]. Similarly, pacidamycin biosynthetic machinery was sufficiently promiscuous to generate new derivatives in better yields than the natural pacidamycin simply by feeding tryptophan analogues to the producing strain [46]. Additionally, by relying on the promiscuity of a cyclooligomer depsipeptide synthase, a library of beauvericins has been generated in a knock-out strain of Beauveria bassiana and by whole cell biocatalysis in E. coli [85].
Rather than applying mutasynthesis by simply deleting biosynthetic functions, several applications are emerging that aim to utilize point mutations to direct the installation of non-natural and non-native extender units. For example, Shultz and coworkers used molecular dynamic simulations to predict a point mutation in the terminal extension module of DEBS that could shift specificity towards propargylmalonyl-thioesters [117]. Indeed, this enzyme-directed mutasynthesis approach led to production of 2-propargyl erythromycin in S. erythraea, although the specificity and product yield of the mutant could not be quantified. In contrast, Williams and colleagues have characterized a series of mutations to the DEBS terminal module that dramatically shift specificity towards non-natural and non-native extender units, enabling the in vitro generation of regioselectively modified macrolactones by utilizing chemically synthesized chain elongation intermediates (Koryakina I, Sherman DH, Williams GJ et al., unpublished results). In principle, these minimally invasive approaches to engineering PKS AT domains could overcome the poor yields often associated with AT-swapping studies. Ultimately though, more sterically or stereoelectronically challenging extender units might require engineering other PKS domains, in addition to the AT. The relatively modest structural differences between various extender units naturally available to PKSs have likely resulted in the distribution of subtle specificity determinants across a large number of AT residues. Subsequently, although various AT motifs can be used to predict extender unit specificity quite well, these motifs have yet to be utilized in the same efficiency that NRPS A domain specificity codes have.
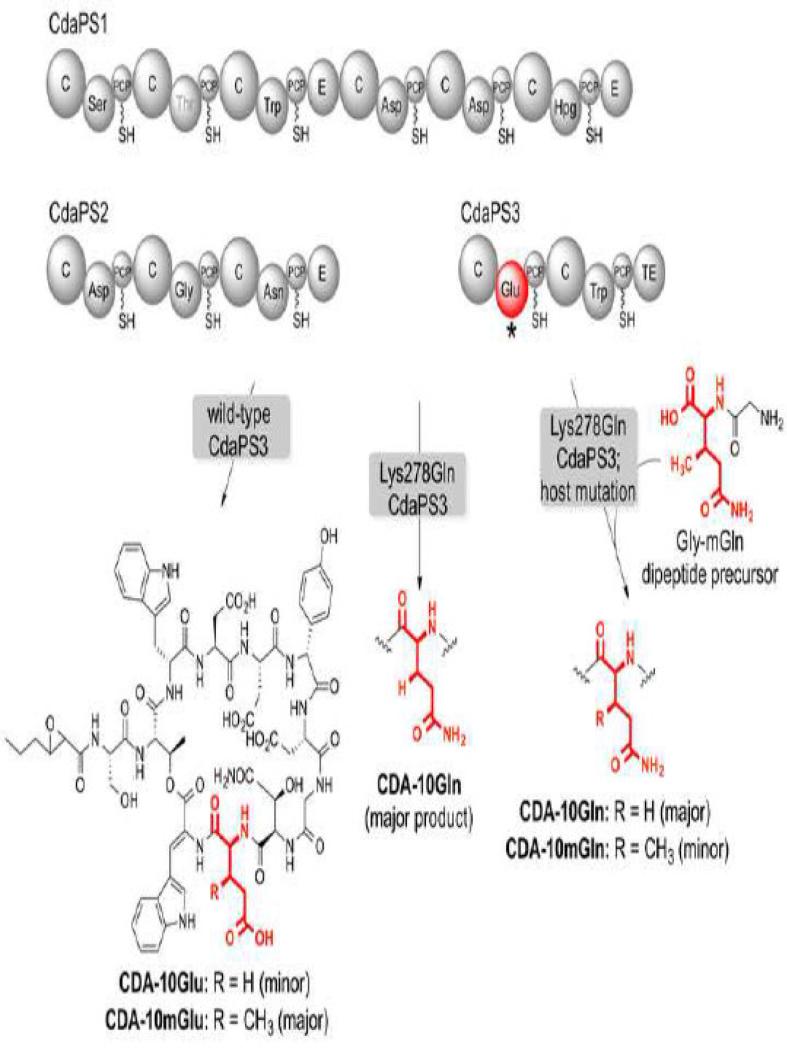
In cases where the inherent substrate promiscuity is insufficient, or where more distantly related non-native substrates are targeted, the amino acid specificity code [112,17] of NRPS A domains has been utilized in minimally invasive engineering efforts [49]. For example, while the natural substrates for module 10 of the calcium-dependent antibiotic (CDA) NRPS are glutamic acid and (2S,3R)-3-methylglutamic acid, a single amino acid mutation was sufficient to generate a novel glutamine-containing CDA analogue in addition to another that included the synthetic non-natural amino acid, (2S,3R)-3-methyl glutamine (Figure 6) [126]. Similarly, guided by comparative in silico analysis of the specificity-conferring code of various NRPS A domains, single and double point mutations were designed to engineer the amino acid specificity of the luminmide NPRS [8]. These mutations were introduced into the NRPS gene directly by recombineering in E. coli, resulting in the production of the desired analogues. A single amino acid mutation in the phenylalanine-specific A domain of the gramicidin S NRPS resulted in a 105-fold switch in substrate specificity towards non-natural aromatic amino acids functionalized with azide and alkyne groups, without loss of catalytic efficiency [75]. In a relatively rare example of assembly line directed evolution, the A-domain of tyrocidine synthetase 1 (TycA) was subjected to combinatorial active site mutagenesis and high-throughput screening [128]. Whereas the preferred substrate of TycA A-domain is L-phenylalanine [129], the very weak promiscuous activity of the wild-type enzyme towards L-Ala was enhanced 170-fold and the specificity (L-Ala vs. L-Phe) shifted by 105-fold. Notably, high-throughput LC-MS/MS was used to screen a library of admK mutants from the andrimid NRPS [38]. By carrying out screening directly in the producing organism, and by searching for novel structures rather than bioactivity, several mutants were identified that supported production of new andrimid analogues. Additionally, a yeast surface display strategy based on affinity binding with bisubstrate analogue probes was employed to identify A-domain mutants of DhbE that displayed improvements in specificity towards several non-natural substrates [153]. The incredible throughput of this approach was leveraged by fully randomizing four active site residues, providing a theoretical library size of 1.6×105. As might be expected, the best mutants were improved in terms of Kms with the non-native substrates. Recently, several examples have emerged that leveraged NRPS subdomains to engineer significant changes in specificity towards substrates very different from the native amino acids [74,28]. Computational structure-based redesign of A domain specificity has also been reported [22].
Figure 6.
Engineering the extender unit specificity of the CDA NRPS. Mutation of lysine 278 to glutamine shifts the specificity of CdaPS3 from 3-methylglutamate/glutamate to 3-methylglutamine/glutamine, with the 3-methyl derivative being preferred. A glycine-3-methylglutamine dipeptide precursor is proteolyzed during fermentation to provide the source of 3-methylglutamine. Coupled with a host mutation, the precursor and Lys278Glu mutation furnish a novel product, CDA-10mGln.
Other assembly line modifications
Both PKS and NRPS assembly lines can contain a variety of optional domains for inline tailoring of the growing natural product chain. Most of the focus has been directed at the reductive domains through either domain swapping or mutagenesis. Whole PKS reductive loops have been successfully swapped to alter the stereochemical outcome of a truncated product from a bimodular PKS [67,3]. Using similar bimodular PKSs for screening, site-directed mutagenesis of the amphotericin ketoreductase afforded a change in stereochemistry [155]. In all cases the yields of the desired products varied, which is possibly due to the reduced catalytic efficiency of the altered KR. A reductive domain of an NRPS was also targeted for mutagenesis. Using the crystal structure of the terminal reductase domain from the myxalamide pathway, rational mutations were made to the active site, which improved the wild type activity [7]. Interestingly, the mutations improved activity toward several non-natural alcohols several fold. Other domains, such as the methyltransferase (MT) domain, have potential to diversify assembly line natural products. For example, Tang and coworkers were able to install an alkyne moiety onto chaetoviridin via an unnatural selenium S-adenosyl-L-methionine mimic [139]. This strategy has the potential to install new chemical diversity in other MT-containing assembly lines.
Macrocyclization
The bioactivity of many polyketides and non-ribosomal peptides depends on post assembly line macrocyclization, which is often catalyzed by a C-terminal thioesterase domain (TE) or condensation–like domain (CT) [70,54]. Though the structure and mechanism of several TEs have been determined [15], and a potential high-throughput screen is available for at least one NRPS TE [45], no progress has been made with respect to engineering these enzymes. This is perhaps related to the difficulty of accessing precursors that can be used to probe the specificity of TEs. In addition, a wealth of in vivo and in vitro data suggests a complex and varied picture of substrate specificity and requirements for macrocyclization that depend on the type of TE [54]. Further insight and engineering of TEs could lead to new strategies for cyclizing new or altered polyketides and non-ribosomal peptide scaffolds.
FUTURE PERSPECTIVES
Approaches that rely on substrate promiscuity of assembly line components as a platform for precursor directed biosynthesis and mutagenesis are gaining prominence over approaches that involve chimera design. Regardless of the overall approach taken to engineer assembly lines, three broad areas of focus are likely to impact our ability to engineer the activity and specificity of PKSs and NRPSs.
Firstly, the discovery of novel natural products and associated biosynthetic machinery through genome mining and metagenomics is likely to play a significant role. Bioinformatics platforms such as antiSMASH allow for natural product structures to be easily predicted from genome sequences [133]. The large number of microbes that cannot be cultured has motivated advances in metagenomics [11,90] and activity-based screening [125,124]. These approaches and others offer the potential to discover assembly line parts with useful substrate promiscuity or orthogonal specificities, or even parts with new catalytic mechanisms and chemistry [83,13,106].
Secondly, tools and techniques from synthetic biology need to continue to be applied to natural product assembly lines [68]. For example, combinatorial gene cluster refactoring [110] and gene assembly methods could provide simple plug and play devices [86,146] to rapidly generate mutant or hybrid assembly lines [89]. Inspiration for these approaches may come from examples of the rationally redesigned aureothin pathway to produce luteoreticulin [116]. Further, heterologous hosts could be developed as platforms for production of natural products using engineered assembly lines [82]. Genome editing technologies such as CRISPR-Cas9 will enable fast and efficient engineering of host strains [127,25].
Thirdly, new strategies for engineering the activity and substrate specificity of assembly lines need to be developed. Molecular dynamics simulations are emerging as a powerful approach to understand the specificity of enzymes related to assembly lines [95] and tailoring enzymes [96,61]. However, while it remains to be seen whether such in silico methods can be applied to understand and alter the specificity of PKS/NRPS assembly lines themselves, the complex dynamics and protein-protein interactions have hampered effective computational modeling. At the same time, the application of evolutionary strategies [29,103] to engineer the activity and specificity of assembly lines has been hindered by a lack of suitable high-throughput screens. Clever systems based on yeast cell surface display [23,153] or perhaps PACE [30] offer the potential to screen the activity of very large combinatorial libraries of mutants. Yet, these platforms need to be adapted to a wide variety of assembly line functions, and evolved components need to be tested in the context of the complete assembly line. High-throughput screens or selections that detect the final product of an assembly line pathway will allow the identification of mutants that are able to process non-natural substrates to the fully mature natural product. Notably, although selections have been used to guide the engineering of chimeric assembly lines and pathways [80,81,39], this approach requires that the distinct antibiotic activity of the desired natural product(s) is already known. Genetically encoded biosensors [31] may prove useful general tools for screening libraries of mutant or chimeric assemble lines. For example, an evolved transcription factor biosensor based on AraC was used to screen a library of 2-pyrone synthase mutants, resulting in the identification of a variant that displayed a 19-fold improvement in catalytic efficiency [119]. Genetically encoded biosensors with tailored ligand specificities towards medicinally relevant natural products could have a unifying vertical impact on assembly line engineering and synthetic biology. For example, biosensors that respond to a desired target polyketide or non-ribosomal peptide could enable (1) high-throughput approaches to metabolic engineering [105], (2) combinatorial gene cluster refactoring [110] in a variety of hosts, (3) directed evolution of assembly lines, tailoring enzymes, or entire pathways, and (4) identification of novel enzyme functions from metagenomic libraries.
SUMMARY
In summary, advances in structural and mechanistic enzymology have provided much insight into the function of and ability to engineer PKS and NRPS assembly lines. In the absence of structure, our understanding of the complex protein-protein interactions orchestrated by assembly lines is beginning to improve via the application of chemical tools. With emerging knowledge in hand, the potential modularity of assembly line components might be enhanced. In the meantime, strategies that leverage the substrate promiscuity of assembly line components continue to enjoy success. Advances in high-throughput screening, in silico modeling, and synthetic biology approaches will be the catalyst for engineering assembly lines and diversifying natural products. Ultimately, the future of natural product diversification and the discovery of new therapeutics using biosynthetic assembly lines are highly promising.
ACKNOWLEDGMENTS
Research on natural product chemical and synthetic biology in our lab is supported by a National Science Foundation CAREER Award (CHE-1151299) and NIH grant 1R01GM104258-01. The authors would like to thank Edward Kalkreuter for critically reading this manuscript.
REFERENCES
- 1.Abe I, Morita H. Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat Prod Rep. 2010;27(6):809–838. doi: 10.1039/b909988n. doi:10.1039/b909988n. [DOI] [PubMed] [Google Scholar]
- 2.Alexander DC, Rock J, Gu JQ, Mascio C, Chu M, Brian P, Baltz RH. Production of novel lipopeptide antibiotics related to A54145 by Streptomyces fradiae mutants blocked in biosynthesis of modified amino acids and assignment of lptJ, lptK and lptL gene functions. J Antibiot (Tokyo) 2011;64(1):79–87. doi: 10.1038/ja.2010.138. doi:10.1038/ja.2010.138. [DOI] [PubMed] [Google Scholar]
- 3.Annaval T, Paris C, Leadlay PF, Jacob C, Weissman KJ. Evaluating ketoreductase exchanges as a means of rationally altering polyketide stereochemistry. ChemBioChem. 2015;16(9):1357–1364. doi: 10.1002/cbic.201500113. doi:10.1002/cbic.201500113. [DOI] [PubMed] [Google Scholar]
- 4.Ashley GW, Burlingame M, Desai R, Fu H, Leaf T, Licari PJ, Tran C, Abbanat D, Bush K, Macielag M. Preparation of erythromycin analogs having functional groups at C-15. J Antibiot (Tokyo) 2006;59(7):392–401. doi: 10.1038/ja.2006.56. doi:10.1038/ja.2006.56. [DOI] [PubMed] [Google Scholar]
- 5.Baerga-Ortiz A, Popovic B, Siskos AP, O'Hare HM, Spiteller D, Williams MG, Campillo N, Spencer JB, Leadlay PF. Directed mutagenesis alters the stereochemistry of catalysis by isolated ketoreductase domains from the erythromycin polyketide synthase. Chem Biol. 2006;13(3):277–285. doi: 10.1016/j.chembiol.2006.01.004. doi:10.1016/j.chembiol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Baltz RH. Combinatorial biosynthesis of cyclic lipopeptide antibiotics: a model for synthetic biology to accelerate the evolution of secondary metabolite biosynthetic pathways. ACS Synth Biol. 2014;3(10):748–758. doi: 10.1021/sb3000673. doi:10.1021/sb3000673. [DOI] [PubMed] [Google Scholar]
- 7.Barajas JF, Phelan RM, Schaub AJ, Kliewer JT, Kelly PJ, Jackson DR, Luo R, Keasling JD, Tsai SC. Comprehensive structural and biochemical analysis of the terminal myxalamid reductase domain for the engineered production of primary alcohols. Chem Biol. 2015 doi: 10.1016/j.chembiol.2015.06.022. doi:10.1016/j.chembiol.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Bian X, Plaza A, Yan F, Zhang Y, Muller R. Rational and efficient site-directed mutagenesis of adenylation domain alters relative yields of luminmide derivatives in vivo. Biotechnol Bioeng. 2015;112(7):1343–1353. doi: 10.1002/bit.25560. doi:10.1002/bit.25560. [DOI] [PubMed] [Google Scholar]
- 9.Bloudoff K, Rodionov D, Schmeing TM. Crystal structures of the first condensation domain of CDA synthetase suggest conformational changes during the synthetic cycle of nonribosomal peptide synthetases. J Mol Biol. 2013;425(17):3137–3150. doi: 10.1016/j.jmb.2013.06.003. doi:10.1016/j.jmb.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boettger D, Hertweck C. Molecular diversity sculpted by fungal PKS-NRPS hybrids. ChemBioChem. 2013;14(1):28–42. doi: 10.1002/cbic.201200624. doi:10.1002/cbic.201200624. [DOI] [PubMed] [Google Scholar]
- 11.Brady SF, Simmons L, Kim JH, Schmidt EW. Metagenomic approaches to natural products from free-living and symbiotic organisms. Nat Prod Rep. 2009;26(11):1488–1503. doi: 10.1039/b817078a. doi:10.1039/b817078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bravo-Rodriguez K, Ismail-Ali AF, Klopries S, Kushnir S, Ismail S, Fansa EK, Wittinghofer A, Schulz F, Sanchez-Garcia E. Predicted incorporation of non-native substrates by a polyketide synthase yields bioactive natural product derivatives. ChemBioChem. 2014;15(13):1991–1997. doi: 10.1002/cbic.201402206. doi:10.1002/cbic.201402206. [DOI] [PubMed] [Google Scholar]
- 13.Bretschneider T, Heim JB, Heine D, Winkler R, Busch B, Kusebauch B, Stehle T, Zocher G, Hertweck C. Vinylogous chain branching catalysed by a dedicated polyketide synthase module. Nature. 2013;502(7469):124–128. doi: 10.1038/nature12588. doi:10.1038/nature12588. [DOI] [PubMed] [Google Scholar]
- 14.Brown S, O'Connor S. Halogenase engineering for the generation of novel natural products. ChemBioChem. 2015 doi: 10.1002/cbic.201500338. doi:10.1002/cbic.201500338. [DOI] [PubMed] [Google Scholar]
- 15.Bruner SD, Weber T, Kohli RM, Schwarzer D, Marahiel MA, Walsh CT, Stubbs MT. Structural basis for the cyclization of the lipopeptide antibiotic surfactin by the thioesterase domain SrfTE. Structure. 2002;10(3):301–310. doi: 10.1016/s0969-2126(02)00716-5. [DOI] [PubMed] [Google Scholar]
- 16.Buchholz TJ, Geders TW, Bartley FE, 3rd, Reynolds KA, Smith JL, Sherman DH. Structural basis for binding specificity between subclasses of modular polyketide synthase docking domains. ACS Chem Biol. 2009;4(1):41–52. doi: 10.1021/cb8002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Challis GL, Ravel J, Townsend CA. Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem Biol. 2000;7(3):211–224. doi: 10.1016/s1074-5521(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 18.Chan YA, Podevels AM, Kevany BM, Thomas MG. Biosynthesis of polyketide synthase extender units. Nat Prod Rep. 2009;26(1):90. doi: 10.1039/b801658p. doi:10.1039/b801658p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang C, Huang R, Yan Y, Ma H, Dai Z, Zhang B, Deng Z, Liu W, Qu X. Uncovering the formation and selection of benzylmalonyl-CoA from the biosynthesis of splenocin and enterocin reveals a versatile way to introduce amino acids into polyketide carbon scaffolds. J Am Chem Soc. 2015;137(12):4183–4190. doi: 10.1021/jacs.5b00728. doi:10.1021/jacs.5b00728. [DOI] [PubMed] [Google Scholar]
- 20.Charkoudian LK, Liu CW, Capone S, Kapur S, Cane DE, Togni A, Seebach D, Khosla C. Probing the interactions of an acyl carrier protein domain from the 6-deoxyerythronolide B synthase. Protein Sci. 2011;20(7):1244–1255. doi: 10.1002/pro.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen AY, Cane DE, Khosla C. Structure-based dissociation of a type I polyketide synthase module. Chem Biol. 2007;14(7):784–792. doi: 10.1016/j.chembiol.2007.05.015. doi:10.1016/j.chembiol.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CY, Georgiev I, Anderson AC, Donald BR. Computational structure-based redesign of enzyme activity. Proc Natl Acad Sci USA. 2009;106(10):3764–3769. doi: 10.1073/pnas.0900266106. doi:10.1073/pnas.0900266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen I, Dorr BM, Liu DR. A general strategy for the evolution of bond-forming enzymes using yeast display. Proc Natl Acad Sci USA. 2011;108(28):11399–11404. doi: 10.1073/pnas.1101046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiocchini C, Linne U, Stachelhaus T. In vivo biocombinatorial synthesis of lipopeptides by COM domain-mediated reprogramming of the surfactin biosynthetic complex. Chem Biol. 2006;13(8):899–908. doi: 10.1016/j.chembiol.2006.06.015. doi:10.1016/j.chembiol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Cobb RE, Wang Y, Zhao H. High-efficiency multiplex genome editing of Streptomyces species using an engineered CRISPR/Cas system. ACS Synth Biol. 2015;4(6):723–728. doi: 10.1021/sb500351f. doi:10.1021/sb500351f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox RJ, Simpson TJ. Fungal type I polyketide synthases. Methods Enzymol. 2009;459:49–78. doi: 10.1016/S0076-6879(09)04603-5. [DOI] [PubMed] [Google Scholar]
- 27.Crosby J, Crump MP. The structural role of the carrier protein — active controller or passive carrier. Nat Prod Rep. 2012;29(10):1111–1137. doi: 10.1039/c2np20062g. doi:10.1039/c2np20062g. [DOI] [PubMed] [Google Scholar]
- 28.Crusemann M, Kohlhaas C, Piel J. Evolution-guided engineering of nonribosomal peptide synthetase adenylation domains. Chem Sci. 2013;4:1041–1045. [Google Scholar]
- 29.Denard CA, Ren H, Zhao H. Improving and repurposing biocatalysts via directed evolution. Curr Opin Chem Biol. 2015;25:55–64. doi: 10.1016/j.cbpa.2014.12.036. doi:10.1016/j.cbpa.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Dickinson BC, Packer MS, Badran AH, Liu DR. A system for the continuous directed evolution of proteases rapidly reveals drug-resistance mutations. Nat Commun. 2014;5:5352. doi: 10.1038/ncomms6352. doi:10.1038/ncomms6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietrich JA, McKee AE, Keasling JD. High-throughput metabolic engineering: advances in small-molecule screening and selection. Annu Rev Biochem. 2010;79:563–590. doi: 10.1146/annurev-biochem-062608-095938. doi:10.1146/annurev-biochem-062608-095938. [DOI] [PubMed] [Google Scholar]
- 32.Dunn BJ, Khosla C. Engineering the acyltransferase substrate specificity of assembly line polyketide synthases. J R Soc Interface. 2013;10(85):20130297. doi: 10.1098/rsif.2013.0297. doi:10.1098/rsif.2013.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta S, Whicher JR, Hansen DA, Hale WA, Chemler JA, Congdon GR, Narayan AR, Hakansson K, Sherman DH, Smith JL, Skiniotis G. Structure of a modular polyketide synthase. Nature. 2014;510(7506):512–517. doi: 10.1038/nature13423. doi:10.1038/nature13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutton CJ, Gibson SP, Goudie AC, Holdom KS, Pacey MS, Ruddock JC, Bu'Lock JD, Richards MK. Novel avermectins produced by mutational biosynthesis. J Antibiot (Tokyo) 1991;44(3):357–365. doi: 10.7164/antibiotics.44.357. [DOI] [PubMed] [Google Scholar]
- 35.Edwards AL, Matsui T, Weiss TM, Khosla C. Architectures of whole-module and bimodular proteins from the 6-deoxyerythronolide B synthase. J Mol Biol. 2014;426(11):2229–2245. doi: 10.1016/j.jmb.2014.03.015. doi:10.1016/j.jmb.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emmert EAB, Klimowicz AK, Thomas MG, Handelsman J. Genetics of zwittermicin A production by Bacillus cereus. Appl Environ Microbiol. 2004;70(1):104–113. doi: 10.1128/AEM.70.1.104-113.2004. doi:10.1128/aem.70.1.104-113.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eustaquio AS, Moore BS. Mutasynthesis of fluorosalinosporamide, a potent and reversible inhibitor of the proteasome. Angew Chem Int Ed Engl. 2008;47(21):3936–3938. doi: 10.1002/anie.200800177. doi:10.1002/anie.200800177. [DOI] [PubMed] [Google Scholar]
- 38.Evans BS, Chen Y, Metcalf WW, Zhao H, Kelleher NL. Directed evolution of the nonribosomal peptide synthetase AdmK generates new andrimid derivatives in vivo. Chem Biol. 2011;18(5):601–607. doi: 10.1016/j.chembiol.2011.03.008. doi:10.1016/j.chembiol.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischbach MA, Lai JR, Roche ED, Walsh CT, Liu DR. Directed evolution can rapidly improve the activity of chimeric assembly-line enzymes. Proc Natl Acad Sci USA. 2007;104(29):11951–11956. doi: 10.1073/pnas.0705348104. doi:10.1073/pnas.0705348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gantt RW, Peltier-Pain P, Thorson JS. Enzymatic methods for glyco(diversification/randomization) of drugs and small molecules. Nat Prod Rep. 2011;28(11):1811–1853. doi: 10.1039/c1np00045d. doi:10.1039/c1np00045d. [DOI] [PubMed] [Google Scholar]
- 41.Gay DC, Gay G, Axelrod AJ, Jenner M, Kohlhaas C, Kampa A, Oldham NJ, Piel J, Keatinge-Clay AT. A close look at a ketosynthase from a trans-acyltransferase modular polyketide synthase. Structure. 2014;22(3):444–451. doi: 10.1016/j.str.2013.12.016. doi:10.1016/j.str.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gay DC, Spear PJ, Keatinge-Clay AT. A double-hotdog with a new trick: structure and mechanism of the trans-acyltransferase polyketide synthase enoyl-isomerase. ACS Chem Biol. 2014;9(10):2374–2381. doi: 10.1021/cb500459b. doi:10.1021/cb500459b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goss RJ, Hong H. A novel fluorinated erythromycin antibiotic. Chem Commun (Camb) 2005;(31):3983–3985. doi: 10.1039/b506635b. doi:10.1039/b506635b. [DOI] [PubMed] [Google Scholar]
- 44.Goss RJ, Lanceron S, Deb Roy A, Sprague S, Nur EAM, Hughes DL, Wilkinson B, Moss SJ. An expeditious route to fluorinated rapamycin analogues by utilising mutasynthesis. ChemBioChem. 2010;11(5):698–702. doi: 10.1002/cbic.200900723. doi:10.1002/cbic.200900723. [DOI] [PubMed] [Google Scholar]
- 45.Grunewald J, Kopp F, Mahlert C, Linne U, Sieber SA, Marahiel MA. Fluorescence resonance energy transfer as a probe of peptide cyclization catalyzed by nonribosomal thioesterase domains. Chem Biol. 2005;12(8):873–881. doi: 10.1016/j.chembiol.2005.05.019. doi:10.1016/j.chembiol.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Gruschow S, Rackham EJ, Elkins B, Newill PL, Hill LM, Goss RJ. New pacidamycin antibiotics through precursor-directed biosynthesis. ChemBioChem. 2009;10(2):355–360. doi: 10.1002/cbic.200800575. doi:10.1002/cbic.200800575. [DOI] [PubMed] [Google Scholar]
- 47.Hahn M, Stachelhaus T. Selective interaction between nonribosomal peptide synthetases is facilitated by short communication-mediating domains. Proc Natl Acad Sci USA. 2004;101(44):15585–15590. doi: 10.1073/pnas.0404932101. doi:10.1073/pnas.0404932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahn M, Stachelhaus T. Harnessing the potential of communication-mediating domains for the biocombinatorial synthesis of nonribosomal peptides. Proc Natl Acad Sci USA. 2006;103(2):275–280. doi: 10.1073/pnas.0508409103. doi:10.1073/pnas.0508409103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han JW, Kim EY, Lee JM, Kim YS, Bang E, Kim BS. Site-directed modification of the adenylation domain of the fusaricidin nonribosomal peptide synthetase for enhanced production of fusaricidin analogs. Biotechnol Lett. 2012;34(7):1327–1334. doi: 10.1007/s10529-012-0913-8. doi:10.1007/s10529-012-0913-8. [DOI] [PubMed] [Google Scholar]
- 50.Hans M, Hornung A, Dziarnowski A, Cane DE, Khosla C. Mechanistic analysis of acyl transferase domain exchange in polyketide synthase modules. J Am Chem Soc. 2003;125(18):5366–5374. doi: 10.1021/ja029539i. [DOI] [PubMed] [Google Scholar]
- 51.Harvey CJ, Puglisi JD, Pande VS, Cane DE, Khosla C. Precursor directed biosynthesis of an orthogonally functional erythromycin analogue: selectivity in the ribosome macrolide binding pocket. J Am Chem Soc. 2012;134(29):12259–12265. doi: 10.1021/ja304682q. doi:10.1021/ja304682q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haydock SF, Aparicio JF, Molnar I, Schwecke T, Khaw LE, Konig A, Marsden AF, Galloway IS, Staunton J, Leadlay PF. Divergent sequence motifs correlated with the substrate specificity of (methyl)malonyl-CoA:acyl carrier protein transacylase domains in modular polyketide synthases. FEBS Lett. 1995;374(2):246–248. doi: 10.1016/0014-5793(95)01119-y. [DOI] [PubMed] [Google Scholar]
- 53.Hertweck C, Luzhetskyy A, Rebets Y, Bechthold A. Type II polyketide synthases: gaining a deeper insight into enzymatic teamwork. Nat Prod Rep. 2007;24(1):162–190. doi: 10.1039/b507395m. doi:10.1039/b507395m. [DOI] [PubMed] [Google Scholar]
- 54.Horsman ME, Hari TP, Boddy CN. Polyketide synthase and non-ribosomal peptide synthetase thioesterase selectivity: logic gate or a victim of fate? Nat Prod Rep. 2015 doi: 10.1039/c4np00148f. doi:10.1039/c4np00148f. [DOI] [PubMed] [Google Scholar]
- 55.Hughes AJ, Keatinge-Clay A. Enzymatic extender unit generation for in vitro polyketide synthase reactions: structural and functional showcasing of Streptomyces coelicolor MatB. Chem Biol. 2011;18(2):165–176. doi: 10.1016/j.chembiol.2010.12.014. doi:10.1016/j.chembiol.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 56.Huitt-Roehl CR, Hill EA, Adams MM, Vagstad AL, Li JW, Townsend CA. Starter unit flexibility for engineered product synthesis by the nonreducing polyketide synthase PksA. ACS Chem Biol. 2015;10(6):1443–1449. doi: 10.1021/acschembio.5b00005. doi:10.1021/acschembio.5b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hur GH, Meier JL, Baskin J, Codelli JA, Bertozzi CR, Marahiel MA, Burkart MD. Crosslinking studies of protein-protein interactions in nonribosomal peptide biosynthesis. Chem Biol. 2009;16(4):372–381. doi: 10.1016/j.chembiol.2009.02.009. doi:10.1016/j.chembiol.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hur GH, Vickery CR, Burkart MD. Explorations of catalytic domains in non-ribosomal peptide synthetase enzymology. Nat Prod Rep. 2012;29(10):1074–1098. doi: 10.1039/c2np20025b. doi:10.1039/c2np20025b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishikawa F, Haushalter RW, Lee DJ, Finzel K, Burkart MD. Sulfonyl 3-alkynyl pantetheinamides as mechanism-based cross-linkers of acyl carrier protein dehydratase. J Am Chem Soc. 2013;135(24):8846–8849. doi: 10.1021/ja4042059. doi:10.1021/ja4042059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobsen JR, Hutchinson CR, Cane DE, Khosla C. Precursor-directed biosynthesis of erythromycin analogs by an engineered polyketide synthase. Science. 1997;277(5324):367–369. doi: 10.1126/science.277.5324.367. [DOI] [PubMed] [Google Scholar]
- 61.Jimenez-Oses G, Osuna S, Gao X, Sawaya MR, Gilson L, Collier SJ, Huisman GW, Yeates TO, Tang Y, Houk KN. The role of distant mutations and allosteric regulation on LovD active site dynamics. Nat Chem Biol. 2014;10(6):431–436. doi: 10.1038/nchembio.1503. doi:10.1038/nchembio.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jurjens G, Kirschning A, Candito DA. Lessons from the synthetic chemist nature. Nat Prod Rep. 2015;32(5):723–737. doi: 10.1039/c4np00160e. doi:10.1039/c4np00160e. [DOI] [PubMed] [Google Scholar]
- 63.Kapur S, Chen AY, Cane DE, Khosla C. Molecular recognition between ketosynthase and acyl carrier protein domains of the 6-deoxyerythronolide B synthase. Proc Natl Acad Sci USA. 2010;107(51):22066–22071. doi: 10.1073/pnas.1014081107. doi:10.1073/pnas.1014081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kapur S, Lowry B, Yuzawa S, Kenthirapalan S, Chen AY, Cane DE, Khosla C. Reprogramming a module of the 6-deoxyerythronolide B synthase for iterative chain elongation. Proc Natl Acad Sci USA. 2012;109(11):4110–4115. doi: 10.1073/pnas.1118734109. doi:10.1073/pnas.1118734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keating TA, Marshall CG, Walsh CT, Keating AE. The structure of VibH represents nonribosomal peptide synthetase condensation, cyclization and epimerization domains. Nat Struct Biol. 2002;9(7):522–526. doi: 10.1038/nsb810. doi:10.1038/nsb810. [DOI] [PubMed] [Google Scholar]
- 66.Keatinge-Clay AT. The structures of type I polyketide synthases. Nat Prod Rep. 2012;29(10):1050–1073. doi: 10.1039/c2np20019h. doi:10.1039/c2np20019h. [DOI] [PubMed] [Google Scholar]
- 67.Kellenberger L, Galloway IS, Sauter G, Bohm G, Hanefeld U, Cortes J, Staunton J, Leadlay PF. A polylinker approach to reductive loop swaps in modular polyketide synthases. ChemBioChem. 2008;9(16):2740–2749. doi: 10.1002/cbic.200800332. doi:10.1002/cbic.200800332. [DOI] [PubMed] [Google Scholar]
- 68.Kim E, Moore BS, Yoon YJ. Reinvigorating natural product combinatorial biosynthesis with synthetic biology. Nat Chem Biol. 2015;11(9):649–659. doi: 10.1038/nchembio.1893. doi:10.1038/nchembio.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koglin A, Mofid MR, Lohr F, Schafer B, Rogov VV, Blum MM, Mittag T, Marahiel MA, Bernhard F, Dotsch V. Conformational switches modulate protein interactions in peptide antibiotic synthetases. Science. 2006;312(5771):273–276. doi: 10.1126/science.1122928. doi:10.1126/science.1122928. [DOI] [PubMed] [Google Scholar]
- 70.Kopp F, Marahiel MA. Macrocyclization strategies in polyketide and nonribosomal peptide biosynthesis. Nat Prod Rep. 2007;24(4):735–749. doi: 10.1039/b613652b. doi:10.1039/b613652b. [DOI] [PubMed] [Google Scholar]
- 71.Koryakina I, McArthur J, Randall S, Draelos MM, Musiol EM, Muddiman DC, Weber T, Williams GJ. Poly specific trans-acyltransferase machinery revealed via engineered acyl-CoA synthetases. ACS Chem Biol. 2013;8:200–208. doi: 10.1021/cb3003489. doi:10.1021/cb3003489. [DOI] [PubMed] [Google Scholar]
- 72.Koryakina I, McArthur JB, Draelos MM, Williams GJ. Promiscuity of a modular polyketide synthase towards natural and non-natural extender units. Org Biomol Chem. 2013;11:4449–4458. doi: 10.1039/c3ob40633d. doi:10.1039/c3ob40633d. [DOI] [PubMed] [Google Scholar]
- 73.Koryakina I, Williams GJ. Mutant malonyl-CoA synthetases with altered specificity for polyketide synthase extender unit generation. ChemBioChem. 2011;12(15):2289–2293. doi: 10.1002/cbic.201100383. doi:10.1002/cbic.201100383. [DOI] [PubMed] [Google Scholar]
- 74.Kries H, Niquille DL, Hilvert D. A subdomain swap strategy for reengineering nonribosomal peptides. Chem Biol. 2015;22(5):640–648. doi: 10.1016/j.chembiol.2015.04.015. doi:10.1016/j.chembiol.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 75.Kries H, Wachtel R, Pabst A, Wanner B, Niquille D, Hilvert D. Reprogramming nonribosomal peptide synthetases for “clickable” amino acids. Angew Chem Int Ed Engl. 2014;53(38):10105–10108. doi: 10.1002/anie.201405281. doi:10.1002/anie.201405281. [DOI] [PubMed] [Google Scholar]
- 76.Kuttruff CA, Eastgate MD, Baran PS. Natural product synthesis in the age of scalability. Nat Prod Rep. 2014;31(4):419–432. doi: 10.1039/c3np70090a. doi:10.1039/c3np70090a. [DOI] [PubMed] [Google Scholar]
- 77.Kwan DH, Tosin M, Schlager N, Schulz F, Leadlay PF. Insights into the stereospecificity of ketoreduction in a modular polyketide synthase. Org Biomol Chem. 2011;9(7):2053–2056. doi: 10.1039/c1ob00022e. doi:10.1039/c1ob00022e. [DOI] [PubMed] [Google Scholar]
- 78.Kwon SJ, Mora-Pale M, Lee MY, Dordick JS. Expanding nature's small molecule diversity via in vitro biosynthetic pathway engineering. Curr Opin Chem Biol. 2012;16(1-2):186–195. doi: 10.1016/j.cbpa.2012.02.001. doi:10.1016/j.cbpa.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Lechner A, Wilson MC, Ban YH, Hwang J-y, Yoon YJ, Moore BS. Designed biosynthesis of 36-methyl-FK506 by polyketide precursor pathway engineering. ACS Synth Biol. 2012;2:379–383. doi: 10.1021/sb3001062. doi:10.1021/sb3001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee HY, Harvey CJ, Cane DE, Khosla C. Improved precursor-directed biosynthesis in E. coli via directed evolution. J Antibiot (Tokyo) 2011;64(1):59–64. doi: 10.1038/ja.2010.129. doi:10.1038/ja.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee HY, Khosla C. Bioassay-guided evolution of glycosylated macrolide antibiotics in Escherichia coli. PLoS Biol. 2007;5(2):243–250. doi: 10.1371/journal.pbio.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo Y, Li BZ, Liu D, Zhang L, Chen Y, Jia B, Zeng BX, Zhao H, Yuan YJ. Engineered biosynthesis of natural products in heterologous hosts. Chem Soc Rev. 2015;44(15):5265–5290. doi: 10.1039/c5cs00025d. doi:10.1039/c5cs00025d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma M, Lohman JR, Liu T, Shen B. C-S bond cleavage by a polyketide synthase domain. Proc Natl Acad Sci USA. 2015 doi: 10.1073/pnas.1508437112. doi:10.1073/pnas.1508437112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marahiel MA, Essen LO. Nonribosomal peptide synthetases: mechanistic and structural aspects of essential domains. Methods Enzymol. 2009;458:337–351. doi: 10.1016/S0076-6879(09)04813-7. [DOI] [PubMed] [Google Scholar]
- 85.Matthes D, Richter L, Muller J, Denisiuk A, Feifel SC, Xu Y, Espinosa-Artiles P, Sussmuth RD, Molnar I. In vitro chemoenzymatic and in vivo biocatalytic syntheses of new beauvericin analogues. Chem Commun (Camb) 2012;48(45):5674–5676. doi: 10.1039/c2cc31669b. doi:10.1039/c2cc31669b. [DOI] [PubMed] [Google Scholar]
- 86.Medema MH, Breitling R, Bovenberg R, Takano E. Exploiting plug-and-play synthetic biology for drug discovery and production in microorganisms. Nat Rev Microbiol. 2011;9(2):131–137. doi: 10.1038/nrmicro2478. doi:10.1038/nrmicro2478. [DOI] [PubMed] [Google Scholar]
- 87.Meier JL, Burkart MD. The chemical biology of modular biosynthetic enzymes. Chem Soc Rev. 2009;38(7):2012–2045. doi: 10.1039/b805115c. [DOI] [PubMed] [Google Scholar]
- 88.Menzella HG, Carney JR, Santi DV. Rational design and assembly of synthetic trimodular polyketide synthases. Chem Biol. 2007;14(2):143–151. doi: 10.1016/j.chembiol.2006.12.002. doi:10.1016/j.chembiol.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 89.Menzella HG, Reid R, Carney JR, Chandran SS, Reisinger SJ, Patel KG, Hopwood DA, Santi DV. Combinatorial polyketide biosynthesis by de novo design and rearrangement of modular polyketide synthase genes. Nat Biotechnol. 2005;23(9):1171–1176. doi: 10.1038/nbt1128. [DOI] [PubMed] [Google Scholar]
- 90.Milshteyn A, Schneider JS, Brady SF. Mining the metabiome: identifying novel natural products from microbial communities. Chem Biol. 2014;21(9):1211–1223. doi: 10.1016/j.chembiol.2014.08.006. doi:10.1016/j.chembiol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mo S, Kim DH, Lee JH, Park JW, Basnet DB, Ban YH, Yoo YJ, Chen SW, Park SR, Choi EA, Kim E, Jin YY, Lee SK, Park JY, Liu Y, Lee MO, Lee KS, Kim SJ, Kim D, Park BC, Lee SG, Kwon HJ, Suh JW, Moore BS, Lim SK, Yoon YJ. Biosynthesis of the allylmalonyl-CoA extender unit for the FK506 polyketide synthase proceeds through a dedicated polyketide synthase and facilitates the mutasynthesis of analogues. J Am Chem Soc. 2011;133(4):976–985. doi: 10.1021/ja108399b. doi:10.1021/ja108399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moffet DA, Khosla C, Cane DE. Modular polyketide synthases: Investigating intermodular communication using 6 deoxyerythronolide B synthase module 2. Bioorg Med Chem Lett. 2006;16(1):213–216. doi: 10.1016/j.bmcl.2005.09.017. doi:10.1016/j.bmcl.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 93.Moore BS, Hertweck C. Biosynthesis and attachment of novel bacterial polyketide synthase starter units. Nat Prod Rep. 2002;19(1):70–99. doi: 10.1039/b003939j. [DOI] [PubMed] [Google Scholar]
- 94.Moss SJ, Carletti I, Olano C, Sheridan RM, Ward M, Math V, Nur EAM, Brana AF, Zhang MQ, Leadlay PF, Mendez C, Salas JA, Wilkinson B. Biosynthesis of the angiogenesis inhibitor borrelidin: directed biosynthesis of novel analogues. Chem Commun (Camb) 2006;(22):2341–2343. doi: 10.1039/b602931k. doi:10.1039/b602931k. [DOI] [PubMed] [Google Scholar]
- 95.Mugnai ML, Shi Y, Keatinge-Clay AT, Elber R. Molecular dynamics studies of modular polyketide synthase ketoreductase stereospecificity. Biochemistry. 2015;54(14):2346–2359. doi: 10.1021/bi501401g. doi:10.1021/bi501401g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narayan AR, Jimenez-Oses G, Liu P, Negretti S, Zhao W, Gilbert MM, Ramabhadran RO, Yang YF, Furan LR, Li Z, Podust LM, Montgomery J, Houk KN, Sherman DH. Enzymatic hydroxylation of an unactivated methylene C-H bond guided by molecular dynamics simulations. Nat Chem. 2015;7(8):653–660. doi: 10.1038/nchem.2285. doi:10.1038/nchem.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Newman DJ, Cragg GM. Natural product scaffolds as leads to drugs. Future Med Chem. 2009;1(8):1415–1427. doi: 10.4155/fmc.09.113. [DOI] [PubMed] [Google Scholar]
- 98.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nguyen C, Haushalter RW, Lee DJ, Markwick PR, Bruegger J, Caldara-Festin G, Finzel K, Jackson DR, Ishikawa F, O'Dowd B, McCammon JA, Opella SJ, Tsai SC, Burkart MD. Trapping the dynamic acyl carrier protein in fatty acid biosynthesis. Nature. 2014;505(7483):427–431. doi: 10.1038/nature12810. doi:10.1038/nature12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nguyen KT, He X, Alexander DC, Li C, Gu JQ, Mascio C, Van Praagh A, Mortin L, Chu M, Silverman JA, Brian P, Baltz RH. Genetically engineered lipopeptide antibiotics related to A54145 and daptomycin with improved properties. Antimicrob Agents Chemother. 2010;54(4):1404–1413. doi: 10.1128/AAC.01307-09. doi:10.1128/aac.01307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nigam A, Almabruk KH, Saxena A, Yang J, Mukherjee U, Kaur H, Kohli P, Kumari R, Singh P, Zakharov LN, Singh Y, Mahmud T, Lal R. Modification of rifamycin polyketide backbone leads to improved drug activity against rifampicin-resistant Mycobacterium tuberculosis. J Biol Chem. 2014;289(30):21142–21152. doi: 10.1074/jbc.M114.572636. doi:10.1074/jbc.M114.572636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olano C, Méndez C, Salas JA. Post-PKS tailoring steps in natural product-producing actinomycetes from the perspective of combinatorial biosynthesis. Nat Prod Rep. 2010;27(4):571. doi: 10.1039/b911956f. doi:10.1039/b911956f. [DOI] [PubMed] [Google Scholar]
- 103.Packer MS, Liu DR. Methods for the directed evolution of proteins. Nat Rev Genet. 2015;16(7):379–394. doi: 10.1038/nrg3927. doi:10.1038/nrg3927. [DOI] [PubMed] [Google Scholar]
- 104.Podust LM, Sherman DH. Diversity of P450 enzymes in the biosynthesis of natural products. Nat Prod Rep. 2012;29(10):1251–1266. doi: 10.1039/c2np20020a. doi:10.1039/c2np20020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raman S, Rogers JK, Taylor ND, Church GM. Evolution-guided optimization of biosynthetic pathways. Proc Natl Acad Sci USA. 2014;111(50):17803–17808. doi: 10.1073/pnas.1409523111. doi:10.1073/pnas.1409523111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ross AC, Xu Y, Lu L, Kersten RD, Shao Z, Al-Suwailem AM, Dorrestein PC, Qian PY, Moore BS. Biosynthetic multitasking facilitates thalassospiramide structural diversity in marine bacteria. J Am Chem Soc. 2013;135(3):1155–1162. doi: 10.1021/ja3119674. doi:10.1021/ja3119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rowe CJ, Bohm IU, Thomas IP, Wilkinson B, Rudd BA, Foster G, Blackaby AP, Sidebottom PJ, Roddis Y, Buss AD, Staunton J, Leadlay PF. Engineering a polyketide with a longer chain by insertion of an extra module into the erythromycin-producing polyketide synthase. Chem Biol. 2001;8(5):475–485. doi: 10.1016/s1074-5521(01)00024-2. [DOI] [PubMed] [Google Scholar]
- 108.Schauwecker F, Pfennig F, Grammel N, Keller U. Construction and in vitro analysis of a new bi-modular polypeptide synthetase for synthesis of N-methylated acyl peptides. Chem Biol. 2000;7(4):287–297. doi: 10.1016/s1074-5521(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 109.Schneider A, Stachelhaus T, Marahiel MA. Targeted alteration of the substrate specificity of peptide synthetases by rational module swapping. Mol Gen Genet. 1998;257(3):308–318. doi: 10.1007/s004380050652. [DOI] [PubMed] [Google Scholar]
- 110.Smanski MJ, Bhatia S, Zhao D, Park Y, L BAW, Giannoukos G, Ciulla D, Busby M, Calderon J, Nicol R, Gordon DB, Densmore D, Voigt CA. Functional optimization of gene clusters by combinatorial design and assembly. Nat Biotechnol. 2014;32(12):1241–1249. doi: 10.1038/nbt.3063. doi:10.1038/nbt.3063. [DOI] [PubMed] [Google Scholar]
- 111.Squire CM, Goss RJM, Hong H, Leadlay PF, Staunton J. Catalytically active tetramodular 6-deoxyerythonolide B synthase fusion proteins. ChemBioChem. 2003;4(11):1225–1228. doi: 10.1002/cbic.200300671. [DOI] [PubMed] [Google Scholar]
- 112.Stachelhaus T, Mootz HD, Marahiel MA. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem Biol. 1999;6(8):493–505. doi: 10.1016/S1074-5521(99)80082-9. [DOI] [PubMed] [Google Scholar]
- 113.Staunton J, Caffrey P, Aparicio JF, Roberts GA, Bethell SS, Leadlay PF. Evidence for a double-helical structure for modular polyketide synthases. Nat Struct Biol. 1996;3(2):188–192. doi: 10.1038/nsb0296-188. [DOI] [PubMed] [Google Scholar]
- 114.Staunton J, Weissman KJ. Polyketide biosynthesis: a millennium review. Nat Prod Rep. 2001;18(4):380–416. doi: 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- 115.Struck AW, Thompson ML, Wong LS, Micklefield J. S-adenosyl-methionine-dependent methyltransferases: highly versatile enzymes in biocatalysis, biosynthesis and other biotechnological applications. ChemBioChem. 2012;13(18):2642–2655. doi: 10.1002/cbic.201200556. doi:10.1002/cbic.201200556. [DOI] [PubMed] [Google Scholar]
- 116.Sugimoto Y, Ding L, Ishida K, Hertweck C. Rational design of modular polyketide synthases: morphing the aureothin pathway into a luteoreticulin assembly line. Angew Chem Int Ed Engl. 2014;53(6):1560–1564. doi: 10.1002/anie.201308176. doi:10.1002/anie.201308176. [DOI] [PubMed] [Google Scholar]
- 117.Sundermann U, Bravo-Rodriguez K, Klopries S, Kushnir S, Gomez H, Sanchez-Garcia E, Schulz F. Enzyme-directed mutasynthesis: a combined experimental and theoretical approach to substrate recognition of a polyketide synthase. ACS Chem Biol. 2013;8:443–450. doi: 10.1021/cb300505w. doi:10.1021/cb300505w. [DOI] [PubMed] [Google Scholar]
- 118.Sundlov JA, Shi C, Wilson DJ, Aldrich CC, Gulick AM. Structural and functional investigation of the intermolecular interaction between NRPS adenylation and carrier protein domains. Chem Biol. 2012;19(2):188–198. doi: 10.1016/j.chembiol.2011.11.013. doi:10.1016/j.chembiol.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang SY, Qian S, Akinterinwa O, Frei CS, Gredell JA, Cirino PC. Screening for enhanced triacetic acid lactone production by recombinant Escherichia coli expressing a designed triacetic acid lactone reporter. J Am Chem Soc. 2013;135(27):10099–10103. doi: 10.1021/ja402654z. doi:10.1021/ja402654z. [DOI] [PubMed] [Google Scholar]
- 120.Tang Y, Chen AY, Kim CY, Cane DE, Khosla C. Structural and mechanistic analysis of protein interactions in module 3 of the 6-deoxyerythronolide B synthase. Chem Biol. 2007;14(8):931–943. doi: 10.1016/j.chembiol.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang YY, Kim CY, Mathews II, Cane DE, Khosla C. The 2.7-angstrom crystal structure of a 194-kDa homodimeric fragment of the 6-deoxyerythronolide B synthase. Proc Natl Acad Sci U S A. 2006;103(30):11124–11129. doi: 10.1073/pnas.0601924103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tanovic A, Samel SA, Essen LO, Marahiel MA. Crystal structure of the termination module of a nonribosomal peptide synthetase. Science. 2008;321(5889):659–663. doi: 10.1126/science.1159850. [DOI] [PubMed] [Google Scholar]
- 123.Tarry MJ, Schmeing TM. Specific disulfide cross-linking to constrict the mobile carrier domain of nonribosomal peptide synthetases. Protein Eng Des Sel. 2015;28(6):163–170. doi: 10.1093/protein/gzv009. doi:10.1093/protein/gzv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thaker MN, Wang W, Spanogiannopoulos P, Waglechner N, King AM, Medina R, Wright GD. Identifying producers of antibacterial compounds by screening for antibiotic resistance. Nat Biotechnol. 2013;31(10):922–927. doi: 10.1038/nbt.2685. doi:10.1038/nbt.2685. [DOI] [PubMed] [Google Scholar]
- 125.Thaker MN, Wright GD. Opportunities for synthetic biology in antibiotics: expanding glycopeptide chemical diversity. ACS Synth Biol. 2012 doi: 10.1021/sb300092n. doi:10.1021/sb300092n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thirlway J, Lewis R, Nunns L, Al Nakeeb M, Styles M, Struck A-W, Smith C, Micklefield J. Introduction of a non-natural amino acid into a nonribosomal peptide antibiotic by modification of adenylation domain specificity. Angew Chem Int Ed Engl. 2012;51(1521-3773 (Electronic)):7181–7184. doi: 10.1002/anie.201202043. [DOI] [PubMed] [Google Scholar]
- 127.Tong Y, Charusanti P, Zhang L, Weber T, Lee SY. CRISPR-Cas9 based engineering of Actinomycetal genomes. ACS Synth Biol. 2015 doi: 10.1021/acssynbio.5b00038. doi:10.1021/acssynbio.5b00038. [DOI] [PubMed] [Google Scholar]
- 128.Villiers B, Hollfelder F. Directed evolution of a gatekeeper domain in nonribosomal peptide synthesis. Chem Biol. 2011;18(10):1290–1299. doi: 10.1016/j.chembiol.2011.06.014. doi:10.1016/j.chembiol.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 129.Villiers BR, Hollfelder F. Mapping the limits of substrate specificity of the adenylation domain of TycA. ChemBioChem. 2009;10(4):671–682. doi: 10.1002/cbic.200800553. [DOI] [PubMed] [Google Scholar]
- 130.Walker MC, Thuronyi BW, Charkoudian LK, Lowry B, Khosla C, Chang MC. Expanding the fluorine chemistry of living systems using engineered polyketide synthase pathways. Science. 2013;341(6150):1089–1094. doi: 10.1126/science.1242345. doi:10.1126/science.1242345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Walsh CT. Insights into the chemical logic and enzymatic machinery of NRPS assembly lines. Nat Prod Rep. 2015 doi: 10.1039/c5np00035a. doi:10.1039/c5np00035a. [DOI] [PubMed] [Google Scholar]
- 132.Wang F, Wang Y, Ji J, Zhou Z, Yu J, Zhu H, Su Z, Zhang L, Zheng J. Structural and functional analysis of the loading acyltransferase from avermectin modular polyketide synthase. ACS Chem Biol. 2015;10(4):1017–1025. doi: 10.1021/cb500873k. doi:10.1021/cb500873k. [DOI] [PubMed] [Google Scholar]
- 133.Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R, Lee SY, Fischbach MA, Muller R, Wohlleben W, Breitling R, Takano E, Medema MH. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43(W1):W237–243. doi: 10.1093/nar/gkv437. doi:10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weissman KJ. Uncovering the structures of modular polyketide synthases. Nat Prod Rep. 2015;32(3):436–453. doi: 10.1039/c4np00098f. doi:10.1039/c4np00098f. [DOI] [PubMed] [Google Scholar]
- 135.Whicher JR, Dutta S, Hansen DA, Hale WA, Chemler JA, Dosey AM, Narayan AR, Hakansson K, Sherman DH, Smith JL, Skiniotis G. Structural rearrangements of a polyketide synthase module during its catalytic cycle. Nature. 2014;510(7506):560–564. doi: 10.1038/nature13409. doi:10.1038/nature13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whicher JR, Smaga SS, Hansen DA, Brown WC, Gerwick WH, Sherman DH, Smith JL. Cyanobacterial polyketide synthase docking domains: a tool for engineering natural product biosynthesis. Chem Biol. 2013;20(11):1340–1351. doi: 10.1016/j.chembiol.2013.09.015. doi:10.1016/j.chembiol.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Williams GJ, Zhang C, Thorson JS. Natural product glycosyltransferases: Properties and applications. Adv Enzymol Relat Areas Mol Biol. 2008;76:55–119. doi: 10.1002/9780470392881.ch2. [DOI] [PubMed] [Google Scholar]
- 138.Wilson MC, Moore BS. Beyond ethylmalonyl-CoA: the functional role of crotonyl-CoA carboxylase/reductase homologs in expanding polyketide diversity. Nat Prod Rep. 2012;29(1):72–86. doi: 10.1039/c1np00082a. doi:10.1039/c1np00082a. [DOI] [PubMed] [Google Scholar]
- 139.Winter JM, Chiou G, Bothwell IR, Xu W, Garg NK, Luo M, Tang Y. Expanding the structural diversity of polyketides by exploring the cofactor tolerance of an inline methyltransferase domain. Org Lett. 2013;15(14):3774–3777. doi: 10.1021/ol401723h. doi:10.1021/ol401723h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wong FT, Jin X, Mathews II, Cane DE, Khosla C. Structure and mechanism of the trans-acting acyltransferase from the disorazole synthase. Biochemistry. 2011;50:6539–6548. doi: 10.1021/bi200632j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Worthington AS, Hur GH, Meier JL, Cheng Q, Moore BS, Burkart MD. Probing the compatibility of type II ketosynthase-carrier protein partners. ChemBioChem. 2008;9(13):2096–2103. doi: 10.1002/cbic.200800198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Worthington AS, Porter DF, Burkart MD. Mechanism-based crosslinking as a gauge for functional interaction of modular synthases. Org Biomol Chem. 2010;8(8):1769–1772. doi: 10.1039/b925966j. [DOI] [PubMed] [Google Scholar]
- 143.Worthington AS, Rivera H, Torpey JW, Alexander MD, Burkart MD. Mechanism-based protein cross-linking probes to investigate carrier protein-mediated biosynthesis. ACS Chem Biol. 2006;1(11):687–691. doi: 10.1021/cb6003965. [DOI] [PubMed] [Google Scholar]
- 144.Wright PM, Seiple IB, Myers AG. The evolving role of chemical synthesis in antibacterial drug discovery. Angew Chem Int Ed Engl. 2014;53(34):8840–8869. doi: 10.1002/anie.201310843. doi:10.1002/anie.201310843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xie Y, Cai Q, Ren H, Wang L, Xu H, Hong B, Wu L, Chen R. NRPS substrate promiscuity leads to more potent antitubercular sansanmycin analogues. J Nat Prod. 2014;77(7):1744–1748. doi: 10.1021/np5001494. doi:10.1021/np5001494. [DOI] [PubMed] [Google Scholar]
- 146.Yamanaka K, Reynolds KA, Kersten RD, Ryan KS, Gonzalez DJ, Nizet V, Dorrestein PC, Moore BS. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc Natl Acad Sci USA. 2014;111(5):1957–1962. doi: 10.1073/pnas.1319584111. doi:10.1073/pnas.1319584111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yan J, Gupta S, Sherman DH, Reynolds KA. Functional dissection of a multimodular polypeptide of the pikromycin polyketide synthase into monomodules by using a matched pair of heterologous docking domains. ChemBioChem. 2009;10(9):1537–1543. doi: 10.1002/cbic.200900098. doi:10.1002/cbic.200900098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yan Y, Chen J, Zhang L, Zheng Q, Han Y, Zhang H, Zhang D, Awakawa T, Abe I, Liu W. Multiplexing of combinatorial chemistry in antimycin biosynthesis: expansion of molecular diversity and utility. Angew Chem Int Ed Engl. 2013;52(47):12308–12312. doi: 10.1002/anie.201305569. doi:10.1002/anie.201305569. [DOI] [PubMed] [Google Scholar]
- 149.Ye Z, Bair M, Desai H, Williams GJ. A photocrosslinking assay for reporting protein interactions in polyketide and fatty acid synthases. Mol Biosyst. 2011;7(11):3152–3156. doi: 10.1039/c1mb05270e. doi:10.1039/c1mb05270e. [DOI] [PubMed] [Google Scholar]
- 150.Ye Z, Musiol EM, Weber T, Williams GJ. Reprogramming acyl carrier protein interactions of an Acyl-CoA promiscuous trans-acyltransferase. Chem Biol. 2014;21(5):636–646. doi: 10.1016/j.chembiol.2014.02.019. doi:10.1016/j.chembiol.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ye Z, Williams GJ. Mapping a ketosynthase:acyl carrier protein binding interface via unnatural amino acid-mediated photo-cross-linking. Biochemistry. 2014;53(48):7494–7502. doi: 10.1021/bi500936u. doi:10.1021/bi500936u. [DOI] [PubMed] [Google Scholar]
- 152.Yonus H, Neumann P, Zimmermann S, May JJ, Marahiel MA, Stubbs MT. Crystal structure of DltA. Implications for the reaction mechanism of non-ribosomal peptide synthetase adenylation domains. J Biol Chem. 2008;283(47):32484–32491. doi: 10.1074/jbc.M800557200. doi:10.1074/jbc.M800557200. [DOI] [PubMed] [Google Scholar]
- 153.Zhang K, Nelson KM, Bhuripanyo K, Grimes KD, Zhao B, Aldrich CC, Yin J. Engineering the substrate specificity of the DhbE adenylation domain by yeast cell surface display. Chem Biol. 2013;20(1):92–101. doi: 10.1016/j.chembiol.2012.10.020. doi:10.1016/j.chembiol.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng J, Fage CD, Demeler B, Hoffman DW, Keatinge-Clay AT. The missing linker: a dimerization motif located within polyketide synthase modules. ACS Chem Biol. 2013;8(6):1263–1270. doi: 10.1021/cb400047s. doi:10.1021/cb400047s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng J, Piasecki SK, Keatinge-Clay AT. Structural studies of an A2-type modular polyketide synthase ketoreductase reveal features controlling alpha-substituent stereochemistry. ACS Chem Biol. 2013;8(9):1964–1971. doi: 10.1021/cb400161g. doi:10.1021/cb400161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhu X, Liu J, Zhang W. De novo biosynthesis of terminal alkyne-labeled natural products. Nat Chem Biol. 2015;11(2):115–120. doi: 10.1038/nchembio.1718. doi:10.1038/nchembio.1718. [DOI] [PubMed] [Google Scholar]