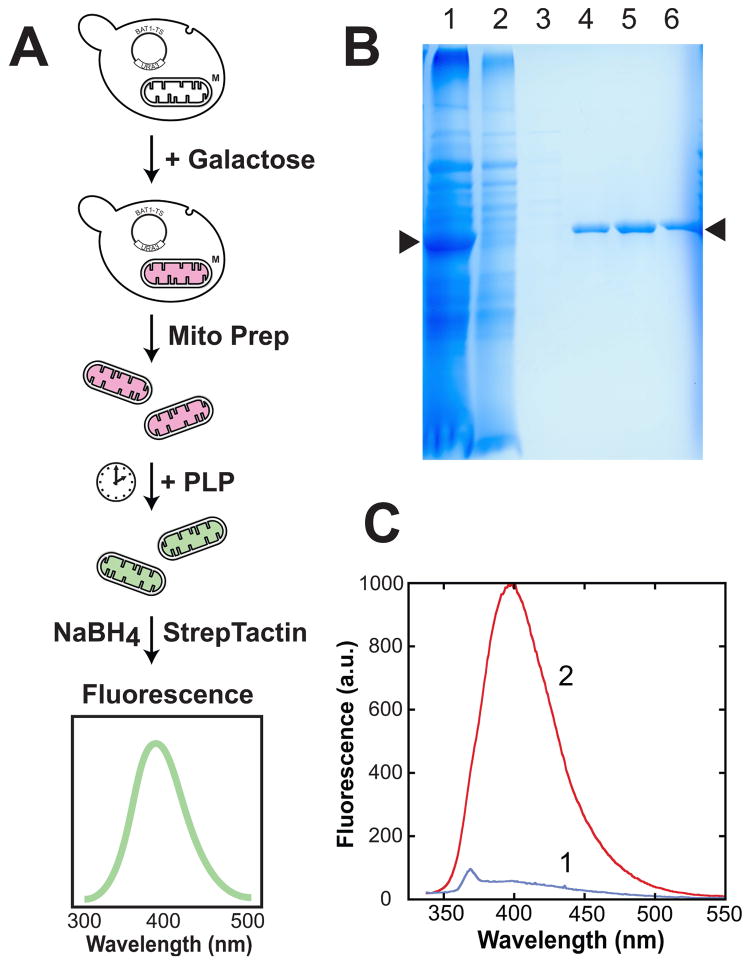
Figure 3.
Loading yeast mitochondria with recombinant Bat1p for in organello assay of PLP transport. (A) Assay scheme: yeast containing an expression vector encoding the mitochondrial branched chain amino transferase (Bat1) fused to a C-terminal TwinStrep affinity tag (pYES2mBAT-TwinStrep) are induced overnight (2% galactose, 2% lactate, 0.2% raffinose) and mitochondria isolated by subcellular fractionation (homogenization, differential centrifugation). Isolated mitochondria are suspended in isosmotic buffer and treated with PLP with rotisserie agitation. A timecourse may be generated by fixing the PLP bound to protein using borohydride reduction at the endpoint. StrepTactin affinity purification of the mBAT-StrepTag protein followed by fluorescence analysis permits quantitation of cofactor content of the protein. (B) Purification of recombinant mBAT-TwinStrep protein from isolated yeast mitochondria. (1) intact isolated mitochondria, showing high level expression of recombinant mBAT-TwinStrep protein; (2) StrepTactin flow-through; (3) StrepTactin wash; (4–6) StrepTactin elution with desthiobiotin. Arrows (▶) identify the location of the recombinant mBAT-TwinStrep protein. (C) Fluorescence spectra for affinity-purified mBAT-TwinStrep isolated from yeast mitochondria before (1) or after (2) incubation with PLP followed by reductive trapping with borohydride in the presence of 1 mM PMSF. Both samples 35 μg/mL protein in 100 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA.