Abstract
The gastrointestinal tract (GIT) is a complex system, which changes in response to requirements of the body. GIT represents a barrier to the external environment. To achieve this, epithelial cells must renew rapidly. This renewal of epithelial cells starts in the fetal life under the influence of many GIT peptides by swallowing amniotic fluid (AF). Development and maturation of GIT is a very complex cascade that begins long before birth and continues during infancy and childhood by breast-feeding. Many factors like genetic preprogramming, local and systemic endocrine secretions and many trophic factors (TF) from swallowed AF contribute and modulate the development and growth of the GIT. GIT morphogenesis, differentiation and functional development depend on the activity of various TF in the AF. This manuscript will review the role of AF borne TF in the development of GIT.
Keywords: Amniotic, Fluid, Gastrointestinal, Factors, Tract, Trophic, Development
Core tip: The gastrointestinal tract (GIT) is a complex system with a combination of factors being responsible for its development. Trophic factors (TF) in amniotic fluid (AF) represent an important component that affects the development and maturation of the GIT during fetal life. We highlight the various phases of GIT development, the formation/circulation of AF, various TF in AF and the respective roles they play in fetal GIT development. We also emphasize that much remains to be known about the milieu of TF within AF. We hope this article provides an insight of what is known about such TF and what we hope to discover in the future.
INTRODUCTION
There is a combination of many factors which are responsible for the growth and development of the gastrointestinal tract (GIT) like genetic programming, trophic factors (TF) in amniotic fluid (AF), endocrine factors like corticosteroids, growth hormone and insulin and paracrine growth factors[1-4]. Development and maturation of GIT is a continuum and a very complex process which finally results in a mature GIT.
As a barrier to the external environment, gut epithelium must be renewed rapidly and repeatedly[1]. Growth and renewal of gut epithelial cells is dependent on controlled cell stimulation and proliferation by a number of signaling processes and TF[1]. The importance of AF in fetal nutrition is an acknowledged fact[5]. It has been documented that swallowing of AF enhanced fetal gastrointestinal (GI) development[5] in rabbits. Experiments performed in sheep showed that esophageal ligation decreases fetal intestinal growth and re-establishment of swallowing promoted growth[5]. Surana et al[6] studied the effect of AF on fetal intestinal growth in humansand and found that proximal obstruction of intestines led to more growth impairment as compared to a distal obstruction. Hirai et al[7] examined the trophic effects of AF, human milk and several recombinant growth factors on a human fetal small intestine cell line. They discovered that AF and human milk promoted cell growth equally[7]. They also noted that growth factors stimulated cell growth but enhancement was less than that of AF alone[7]. All of these suggest that AF is a source of TF for the development and maturation of intestine.
But do we know what a mature GIT is? How can we clinically differentiate immature and mature gut? What affects the maturation and development of GIT during fetal life and after birth? To answer these questions we will start with the development of fetal GIT.
FETAL GIT DEVELOPMENT
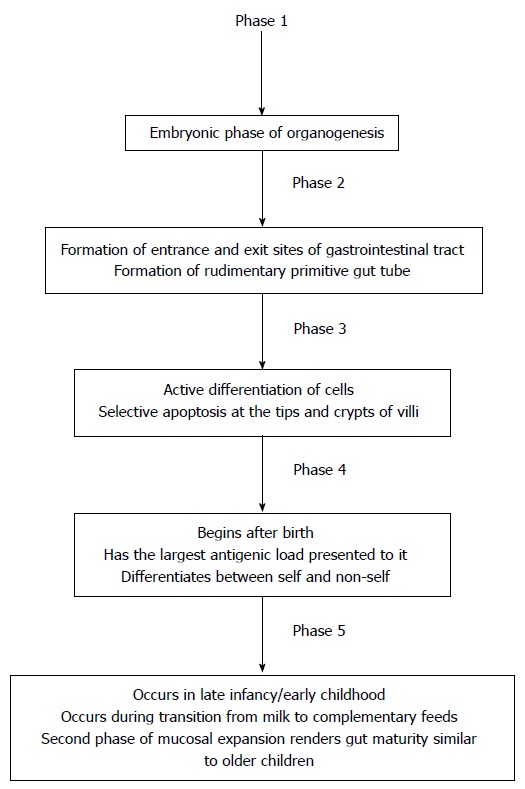
Development and maturation of GIT is a continuous process which starts during early fetal life and continues well into infancy and childhood. Now the question is what a mature GIT is and what leads to a mature GIT? To answer this question, we need to know the fetal and neonatal GIT development and maturation and what is responsible for such processes. There are five phases of fetal GIT development (Table 1 and Figure 1).
Table 1.
Phases of mammalian gastrointestinal development (adapted from reference 1)
| Phase | Development |
| Phase 1 | Embryonic phase of organogenesis |
| Forms primitive GIT | |
| Phase 2 | Entrance and exit sites of GIT form |
| Formation of rudimentary primitive GIT | |
| Formation of mouth and anus | |
| Fetal swallowing of amniotic fluid begins | |
| Phase 3 | Active differentiation |
| Increase in cell number in crypts | |
| Cells from crypts start migrating up to the villi | |
| GIT growth is more rapid than the fetal body as a whole | |
| Growth accompanied by selective apoptosis | |
| Phase 4 | After birth, exposure to enteral nutrition |
| Breast milk feeding – rapid mucosal differentiation and development | |
| Infancy – mucosal growth continues with deepening crypts, increasing villi (increasing width and number) and appearance of sub-mucosal folds | |
| Development of GIT mucosal immunity due to exposure to dietary antigens | |
| Mucosal immune system can distinguish between foreign pathogens and safe nutrient proteins and commensal organisms | |
| Phase 5 (Weaning) | Late infancy – early childhood. Transition from milk feeding to solid foods. This is second phase of mucosal immunity with epithelial hyperplasia with maturation of gut functions similar to older children. |
GIT: Gastrointestinal tract.
Figure 1.
Various phases of mammalian gut development.
Phase 1 (embryonic phase)
This is a phase of organogenesis which begins immediately after conception and quickly leads to phase 2[1]. In phase 1, there is formation of primitive gut. GIT undergoes very rapid growth from the week 5 of gestation onwards[8].
Phase 2
There is selective growth and apoptosis that allows the formation of the rudimentary gut tube[1]. The GIT undergoes rapid growth with the formation of villi and microvilli[8-9]. During this phase an entrance and an exit site is formed (future mouth and anus respectively) and fetus starts swallowing AF, which has both physical and trophic effects on the development of GIT (Figure 2).
Figure 2.

Two patterns of the growth of small intestine. A: Cylindrical organ growth in length and diameter; B: Luminal mucosal growth with amplification of the internal surface area by submucosal folds and villi.
Phase 3 (Late gestational age)
This comprises active differentiation during late gestation when the GIT prepares for extra-uterine life[1]. The intestinal cells actively divide causing cells to migrate up the villus and ultimately forming actively absorbing cells[1]. This phase is also characterized by selective apoptosis at the tips and crypts of villi[10]. After about 96 h, the villous epithelial cells slough off in the lumen of intestine where they mix with mucous and bile to become meconium[9].
Phase 4 (Neonatal phase)
This phase begins after birth when exposure to enteral nutrition leads to more rapid mucosal differentiation and development of GIT for the extra uterine adaptation[1]. Premature infants lack this developmental phase, which occurs during the third trimester. This is why this process is more prolonged in premature infants. This is also the phase that has the largest antigenic load presented to it in the form of dietary proteins and pathogens[1]. During this time the gut develops the ability to distinguish between foreign pathogens and safe nutrient proteins[11,12].
Phase 5 (Weaning phase)
This is the last phase of gut development and occurs in late infancy/early childhood[1] as the child transitions from milk based diet to complementary diet. During this time, there is a second phase of mucosal expansion which is associated with epithelial hyperplasia that renders the gut functionally more mature. During this phase, the intestinal mucosal immunity develops the ability to differentiate between foreign pathogen and nutrient proteins[12]. Preterm infants lack this process due to lack of exposure to AF borne TF due to premature birth. That is why premature infants are at increased risk of proliferation of pathogenic bacteria in the lumen of intestine and subsequent mucosal invasion of pathogenic microorganisms[13]. This may significantly impact the GIT function as well systemic immune functions[13].
FORMATION OF AF
The first fluid to enter the developing fetal GIT is the AF[1]. It is a bioactive medium containing proteins, lipids and phospholipids, urea and electrolytes that is actively secreted by cells lining the amniotic cavity[1]. During early gestation (organogenesis), AF volume increases by getting water from the mother’s plasma and is transported to the fetus through fetal membranes depending on the hydrostatic and osmotic pressure gradients. AF volume increases from 10 mL at 10 wk to around 400 mL at 20 wk gestational age. Around 8-10 wk gestation, the skin is not keratinized hence there is a bi-directional fluid diffusion between the fetus and the AF. During early gestation, AF volume and fetal size are related in a linear manner. Around this time, fetus starts passing urine and fetal swallowing also begins. Keratinization of fetal skin starts around 20 wk of gestation and is completed around 25 wk. After skin keratinization, the relationship of fetal size and AF volume is no longer linear. Around 28 wk gestation, AF volume increases to its maximum volume (about 800 mL) where it plateaus near term gestation and thereafter AF volume starts declining to 400 mL at 42 wk. Fetus swallows around 250 mL/kg/d of AF, which is the main source of AF removal[14]. The chemical composition of AF changes with increasing gestational age. AF is not the main source of fetal nutrition; it contributes only 15% of fetal nutritional requirements[15,16] but has a very important role in the development and maturation of gut[1]. The important nutritional components of AF are summarized in Table 2. In the second half of pregnancy, sodium, chloride and osmolality decrease whereas urea and creatinine concentrations increase. AF composition is more regulated than the AF volume. (Refer to reference 18 for further details)[17].
Table 2.
Important nutritional components of amniotic fluid
| Component | Most important examples |
| Amino acids | Glutamine, arginine |
| Proteins | Lactoferrin |
| Minerals | Zinc, iron |
| Hormones | Growth hormone, prolactin |
| Growth factors | IGF-1, EGF |
IGF-1: Insulin like growth factor-1; EGF: Epidermal growth factor.
AF CIRCULATION
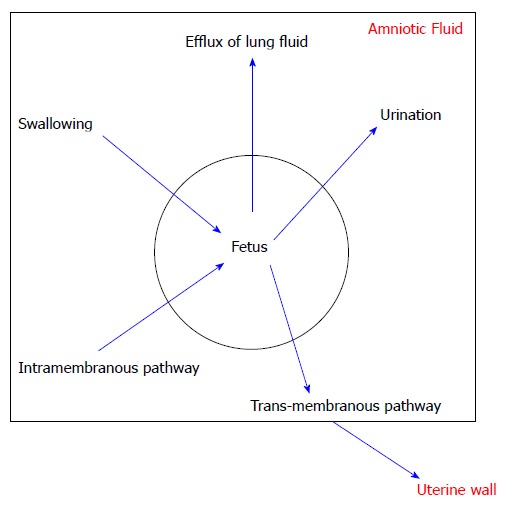
Figure 3 shows 5 pathways of exchange and AF circulation that have been identified[18]. Excretion of urine and the secretion of oral, nasal, tracheal and pulmonary fluids predominantly accomplish production of AF[19]. Fetal breathing movements also lead to the efflux of lung fluid into the AF but this effect is minimal[18]. Removal of AF is predominantly accomplished by fetal swallowing. Also a significant intramembranous pathway transfers fluid and solutes from the amniotic cavity to the fetal circulation across the amniotic membranes[20]. The trans-membranous pathway, which is the movement of AF across the fetal membranes into the maternal circulation, affects the AF volume only minimally[18]. All of these pathways together maintain the relative stability of the AF in spite of large fluid shifts[18].
Figure 3.
Various pathways of amniotic fluid circulation.
VOLUME CHANGES OF AF WITH INCREASING GESTATIONAL AGE
Table 3 summarized the volume changes of AF with increasing gestational age[18].
Table 3.
Amniotic fluid volume changes with increasing gestational age
| Gestational Age | Volume of AF |
| 10 wk | 25 mL |
| 20 wk | 400 mL |
| 28 wk | 800 mL |
| Term gestation | Plateau in volume of AF |
| 42 wk | 400 mL |
AF: Amniotic fluid.
Ten weeks of gestation: 25 mL (AF and fetal size related in a linear fashion in this early period[18]. Fetal kidneys start making urine by 8 wk gestation and swallowing begins soon after).
Twenty weeks of gestation: 400 mL (Keratinization of skin is complete by 25 wk gestation and AF and fetal size lose their linear relationship).
Twenty-eight weeks of gestation: 800 mL.
Term gestation: Plateau near term gestation.
Forty-two weeks of gestation: 400 mL.
AF: SOURCE OF TF
It is important to note that AF is the first fluid which bathes the GIT secondary to swallowing of AF (450 mL/d to 1000 mL/d at term gestation)[21]. Table 4 summarized the roles of various TF found in AF in intestinal development and the location of their receptors. AF serves as the physical barrier to the external environment. In the 1970’s, Chochinov et al[22] correlated AF borne growth hormone concentration with the development and maturation of fetal kidney. Mulvihill et al[16] in vitro studies showed that AF and fetal bovine serum had equivalent stimulating effect on fetal gastric epithelial cells. Further Barka et al[23] confirmed the trophic properties of AF. More recently, Maheshwari[24] described the role of cytokines in AF and their role in the development of GIT. In an in vitro study, Hirai et al[7] demonstrated the trophic effects of AF and further showed that trophic effects of AF were equivalent to breast milk. The growth of intestine occurs by duplication of intestinal crypts, which leads to cylindrical growth of small intestine (Figure 2A). During growth, the crypts gradually divide longitudinally into two daughter crypts (Figure 2B). This process is promoted by various TF like epidermal growth factor (EGF), keratinocyte growth factor, and many other TF which are present in the AF. Over the years, different investigators have found many TF in the AF. Different TF present in AF work in concert to provide bioactivity. Wagner et al[25] used fetal small intestinal cells (Fhs74) and showed a synergistic relationship of EGF and transforming growth factor-α (TGF-α), which was greater than individual effect of recombinant EGF (rEGF), or TGF-α alone. Booth et al[26] also showed that no single growth factor increased cell proliferation of rat intestinal epithelial cells but when rEGF, TGF-α, insulin like growth factor-1 (IGF-1) and platelet derived growth factors were combined, epithelial cell proliferation was increased significantly. How these TF in AF work- whether they work in concert or separately- is only directed by in vitro studies. The interplay of these TF in AF in vivo is not well understood. In this review, we will focus on different TF in the AF and their role in the development and maturation of the GIT.
Table 4.
Roles of various trophic factors found in amniotic fluid in intestinal development and the location of their receptors
| Trophic factor | Location of receptors | Role in intestinal growth |
| EGF | Basolateral intestinal membrane | Stimulates cell mitosis and differentiation |
| Stimulates intestinal epithelial cell proliferation | ||
| HGF | Intestinal crypt epithelial cells and in the muscle layers of the intestine | Intestinal cell proliferation in vitro and has been demonstrated to induce intestinal growth in rats |
| TGF-α and TGF-β | Basolateral intestinal membrane | Primary role may be intestinal mucosal repair |
| IGF-1 | Crypt cells, basolateral membrane and in the distal intestine | Primary mediator of both intrauterine and postnatal growth in mammals |
| May be important for growth of muscle growth of distal small intestine | ||
| EPO | Apical surface of intestinal epithelial cells | Increased villus height, villus area, crypt depth and crypt epithelia cell proliferation in rat pups. In vitro, recombinant EPO has been shown to protect cells against mucosal injury |
| G-CSF | Apical regions of the intestine | Role in epithelial cell maintenance |
| IL family | Intestinal epithelial cells | Enhances intestinal epithelial cell restitution. Enhances the integrity of the intestinal epithelial cell junctions. Intestinal epithelial cell proliferation and increased nutrient uptake |
IGF-1: Insulin like growth factor-1; EGF: Epidermal growth factor; TGF: Transforming growth factor; HGF: Hepatocyte growth factor; EPO: Erythropoietin; G-CSF: Granulocyte colony stimulating factor; IL: Interleukin.
EGF
In 1962, a growth factor was discovered from mouse saliva which could induce premature eruption of the teeth and opening of eyelids - that is why it was called EGF[27]. EGF is a family of peptides that share structure and affinity to the EGF receptor. The salivary glands and Brunner’s glands of duodenum in the GIT continuously secrete EGF. The EGF receptor (erbB1) is found in fetal as well adult GIT, liver and pancreas. EGF receptor levels increase in intestinal pathology like ulceration of rat oxyntic mucosa[28]. It is a small peptide which functions as a luminal surveillance peptide that can attach to the EGF receptor on the basolateral membrane when the luminal barrier is damaged[29]. As GIT is an important barrier to outside noxious substances, there is quick healing of injured epithelial lining by epithelial migration and proliferation, called restitution[30]. EGF stimulates restitution of the superficial epithelial lining of GIT. It stimulates cell mitosis and differentiation, decreases acid secretion, increases bicarbonate, mucus secretions and GIT blood flow and helps in digestion by increasing amylase secretions and by increasing gastric motility. EGF is also a cytoprotective molecule that can stabilize GIT epithelial cells from agents like ethanol or non-steroidal anti-inflammatory drugs[31]. EGF has two main physiological functions: (1) Involved in mucosal protection and healing of damaged epithelial lining; and (2) involved in digestion, absorption and transportation of nutrients.
EGF is found is significant quantities in human AF and it increases with progression of pregnancy[32]. The suggested site of production of EGF is either the lungs or the amniotic membranes[5]. EGF has been shown to increase DNA and glycoprotein synthesis in cultured human fetal gastric cells[33]. The impact of EGF in AF on fetal intestinal growth is an area of active research. EGF receptors are mainly expressed on the basolateral intestinal membrane[24]. It is largely resistant to gastric proteolysis in the preterm infant and thus remains bioavailable in the intestine[24,34]. All in all, EGF is a potent stimulus to intestinal epithelial cell proliferation[15] (Table 5).
Table 5.
Effects of epidermal growth factor on the gastrointestinal tract
| Increased effect on | Possible secondary message |
| Proliferation | - |
| Bicarbonate secretion | Prostaglandins |
| NaCl and glucose uptake | Na+- glucose cotransporter, lipids |
| Mucus secretion | Prostaglandins |
| GI blood flow | Beta-adrenergic NO prostaglandins |
| Longitudinal smooth muscle contraction | Prostaglandins |
| Circular smooth muscle contraction | Desensitizes (not prostaglandins) |
| Restitution | Cell-migration prostaglandins |
| Permeability | - |
| Mucosal protection | Proliferation, polyamines, mucus, trefoil peptides |
| Decreased effect on | Possible secondary message |
| Gastric acid secretion | Protein kinase C, cAMP |
| Gastric emptying | - |
| Increased and decreased effect on | Possible secondary message |
| Chloride secretion | Phosphatidylinositol 3-kinase |
| Pancreatic amylase (3.2.1.1) secretion | cAMP phospholipase C |
GI: Gastrointestinal; NaCl: Sodium chloride; cAMP: Cyclic adenosine monophosphate.
In summary, EGF has mitogenic as well as nonmitogenic roles in GIT function. Further understanding of its role is required before we use it in the settings of inflammation of mucosal damage such as necrotizing enterocolitis.
Hepatocyte growth factor
It is present in AF and human milk and is expressed in embryonic and fetal intestinal tissue[24]. Hepatocyte growth factor (HGF) and C-met mRNA are expressed in the fetal intestine[24]. HGF receptor, C-met - a proto-oncogene, is present on intestinal crypt epithelial cells although it is also expressed in the muscle layers of the intestine[15]. HGF stimulates intestinal cell proliferation in vitro and has been demonstrated to induce intestinal growth in rats when administered in pharmacologic doses[24]. In an animal model of NEC, we showed that oral supplementation of AF is protective against experimental NEC in a rat model of NEC (hypoxia and hypothermia model) which was mediated, at least partly, by HGF.
TGF-α
Detectable in the human GIT at 15 wk gestation[24]. It has a structure similar to EGF and binds to the same receptor. It is found in AF and human milk. Recombinant TGF-α has been shown to elicit a synergistic trophic response on cultured intestinal cells when combined with EGF, IGF-1, FGF and HGF[7]. However it was noted that this trophic response was not as strong as that seen with AF or human milk alone. Its primary role is believed to be in mucosal repair[24].
TGF-beta
It belongs to a family of signaling peptides that influences the distribution of intestinal stem cells. It is found in human AF only during the late stages of gestation[5]. It is believed to induce terminal differentiation of intestinal epithelial cells and to accelerate the rate of healing of intestinal wounds by stimulating cell migration[5]. A role in the prevention of necrotizing enterocolitis has been suggested as well[34]. We showed that TGFβ, especially TGFβ2, suppresses macrophage inflammatory responses in the developing intestine and protects against mucosal inflammatory injury. We further showed that enteral feeding of TGFβ2 protected mice from experimental NEC-like injury[35].
Insulin like IGF-1
It is the primary mediator of both intrauterine and postnatal growth in mammals. Experiments have shown that enterally infused IGF-1 in sheep that have undergone esophageal ligation led to an increase in somatic growth and bowel wall thickness[36]. The concentration of IGF-1 in human AF can reach as high as 20 ng/mL. This means that fetal swallowing near term may lead to 20 mcg of IGF-1 being ingested daily[37].
Insulin like Growth Factor-2
It is a major modulator of early embryonic and 2nd trimester fetal growth[5]. It is synthesized by fetal lung and likely exits the fetal lung via the efflux of fetal lung fluid[5]. Insulin like growth factor-2 (IGF-II) peaks in AF at 19 wk gestation, declining thereafter[38]. Deletion of the IGF-II gene expressed in the placental trophoblast led to reduced placental growth followed several days later by fetal growth restriction[39].
Receptors to IGF-I and IGF-II are expressed on crypt cells, on the basolateral membrane and in the distal intestine[24]. Ingested IGF is likely to remain bioactive in the intestine secondary to its relative stability and presence of milk borne protease inhibitors. Effects similar to the effects on sheep bowel described previously have been observed in human duodenal explants[24].
Fibroblast Growth Factor
Activity has been demonstrated in AF and human milk[7]. A study by Hirai et al[7] showed that inhibition of fibroblast growth factor (FGF) activity in AF caused a 58% reduction in AF induced intestinal epithelial cell proliferation[4], suggesting an important role in the development of the GIT.
Erythropoietin
It is present in AF and human milk and its receptor is present on the apical surface of intestinal epithelial cells[24]. It resists degradation and remains bioactive in the intestine. It has been shown that administration of recombinant erythropoietin (EPO) increased villus height, villus area, crypt depth and crypt epithelial cell proliferation in rat pups[24]. In vitro, recombinant EPO has been shown to protect cells against mucosal injury and there are reports that the incidence of necrotizing enterocolitis is lower in neonates who had received recombinant EPO[40].
Granulocyte colony stimulating factor
Significant concentrations of granulocyte colony stimulating factor (G-CSF) are present in AF and human milk. Similar to EPO, it resists simulated neonatal digestion[24]. Its receptors are mostly present in the apical regions of the intestine starting at 10 wk gestation. There is evidence that G-CSF may play a role in epithelial cell maintenance[41].
Interleukin family
Interleukin (IL)-2 is present in AF and is believed to enhance intestinal epithelial cell restitution. There is evidence that IL-2 plays a crucial role in mucosal healing because IL-2 knockout mice develop colitis similar to human ulcerative colitis[42]. IL-4, which is also present in AF, is believed to enhance the integrity of the intestinal epithelial cell junctions[24]. IL-6 which is detectable in AF as early as 7 wk of gestation, may play a role in protecting intestinal cells against apoptosis secondary to hypoxia or other severe insults[43]. IL-1 found in AF, leads to intestinal epithelial cell proliferation and increased nutrient uptake[24]. IL-1 also induces expression of decay accelerating factor, which is responsible for the degradation of activated complement and thus may play a protective role against complement activation[15].
Other factors such as vascular endothelial growth factor, IL-8 and the trefoil factor family need more in-depth evaluation in terms of their respective roles in the development of the human intestine. Table 6 summarizes the important TF involved in gut development and their important references.
Table 6.
Important trophic factors involved in gut development and the most relevant reference articles
| Trophic factor | Ref. | n of references cited |
| EGF | Maheshwari (2004)[24] | 36 |
| Underwood (2005)[18] | 63 | |
| Playford (1996)[29] | 23 | |
| Cummins (2002)[15] | 108 | |
| HGF | Maheshwari (2004)[24] | 36 |
| Underwood (2005)[18] | 63 | |
| Cummins (2002)[15] | 108 | |
| TGF-α and TGF-β | Maheshwari (2004)[24] | 36 |
| Seare (1998)[56] | 32 | |
| Underwood (2005)[18] | 63 | |
| Cummins (2002)[15] | 108 | |
| IGF-1 | Maheshwari (2004)[24] | 36 |
| Underwood (2005)[18] | 63 | |
| Seare (1998)[56] | 32 | |
| Cummins (2002)[15] | 108 | |
| Cytokines | Maheshwari (2004)[24] | 36 |
| Seare (1998)[56] | 32 |
IGF-1: Insulin like growth factor-1; TGF: Transforming growth factor; EGF: Epidermal growth factor; HGF: Hepatocyte growth factor.
GIT morphogenesis, functional development and differentiation are regulated by various TF present in the AF[44]. In contrast to adult enterocytes, fetal enterocytes hyper-react to microbial challenges hence the defense responses are inadequate[45]. AF borne TF compensates for immaturity of enterocytes by modulating exaggerated responses of the enterocytes and by promoting immune maturation[46]. Enterocytes are not fully mature at birth and breast milk continues to provide TF and immunomodulatory molecules which promote enterocyte development.
EVIDENCE OF AF AS A SOURCE OF TF
Recently, there have been few studies done to show the role of AF in protecting against intestinal mucosal injuries. Good et al[47] showed administration of AF into fetal intestine reduced LPS-mediated signaling within the fetal intestinal mucosa by reducing expression of EGF receptor. The reduced expression of EGF receptor occurs by inhibiting toll-like receptor-4 signaling within the fetal intestine and hence attenuates experimental NEC. Sigger et al[48], in a pig model of NEC showed decreased NEC by enteral administration of AF by suppressing pro-inflammatory intestinal responses. Zani et al[49] demonstrated that intra peritoneal administration of AF stem cells improves survival and reduced NEC like injury in a rat model of NEC. They further showed that pre-treatment with AF stem cells resulted in improved intestinal function, decreased intestinal inflammation and increased enterocyte proliferation. We also showed oral administration of AF protected rat pups against NEC-like mucosal injury, which is partly mediated by HGF[50]. There is more research going in our and other laboratories mainly focusing on the role of each individual TF present in AF by blocking different TF. In summary, there is definite evidence of protective effect of AF against NEC. But still many more questions need to be answered before we can use AF in human infants.
PERSPECTIVE
GIT epithelial biology research has changed to a dynamic interface which is able to sense environmental changes and immune responses. GIT epithelial cells can differentiate even if the stimuli from the gut lumen are harmful or not. This depends upon the underlying mechanisms like immunological tolerance. These functions depend upon the developmental status of the enterocytes. The AF during fetal life and breast milk after birth, compensate for the developmental immaturity to assure the maturation of enterocytes. Understanding of the interaction between fetal AF and exposure to various TF is not only theoretical but is also beginning to modulate the clinical management of premature infants. The use of EGF and other TF present in AF, that target GIT epithelial lining, has expanded a new horizon in the management of premature infants.
APUD (Amine Precursor Uptake and Decarboxylation) cells are responsible for synthesis of biological amines and peptides and accumulation of them in the cytoplasm in the form of secretory granules of “neuroendocrine type”[51]. Originally all APUD cells were believed to be derived from the neural crest. However recently it has been proposed that APUD cells of the GIT originate from neuroendocrine-programmed ectoblast[52]. Andrew et al[53] in 1975 had found APUD cells in the intestinal groove of the chick, disappearing on the day 3 but reappearing by the day 12 onwards. It was hypothesized that the early arising APUD cells are precursors to one or more islet types. It was confirmed in 2007 that AF harbors a heterogeneous mixture of these multipotent stem cells[54]. APUD cells harvested from AF may very well prove to be helpful in the repair of damaged intestinal epithelial cells following disease processes.
LIFE AFTER AF: HUMAN MILK AND THE GIT ADAPTATION
After fetal maturation, human milk is essential for the neonate’s development. Human milk also has the potential to stimulate cell growth and repair and improve immune-competence[55,56]. Human milk contains growth factors, which are similar in nature to that contained in AF. It thus stimulates cells to grow and undergo reparative processes[1]. The neonatal GIT undergoes rapid growth and maturational changes after birth. The tight junctions of the GIT mature and there is selective entrance of nutrients and exclusion of pathogens[57,58]. There is development of an effective mucosal barrier, immaturity of which may result in clinical disease states such as NEC. This intestinal mucous provides a protective interface between the internal and external milieu[1]. Thus a functionally and developmentally mature gut has effective barrier function, epithelial integrity and also adequate mucous production. The role of AF as a source of TF for this above-mentioned development cannot be underscored.
UNANSWERED QUESTIONS
As discussed above, there are so many TF present in AF but the functions and significance of each individual TF remain incompletely understood. Fetal swallowing of AF is very important for the development and maturation of GIT. But some infants are born with esophageal atresia and other conditions leading to malabsorption while other infants have normal functioning GIT. This means there is a way other than just swallowing of AF by which the intestine is exposed to these TF. There is a strong theoretical possibility that TF in AF may protect the preterm infants against NEC and or increase intestinal recovery during the healing phase of NEC[50,59]. Could early feeding of harvested human AF or simulated AF be used in preterm infants at risk for NEC or during recovery phases of NEC? Sullivan et al[60] showed that enteral feeding of “simulated AF” containing G-CSF and EPO was well tolerated in preterm infants and the same group further showed[61] that simulated AF was also tolerated by infants who were recovering from NEC.
Do individual TF work separately or work together in concert? What is the most important TF in AF? Can enough AF be harvested at the time of elective cesarean section deliveries? Would harvested AF remain sterile after storage and safe to be used in preterm infants? Could harvested AF be pasteurized without inactivation of TF? Porter et al[62] have shown stability of TF in AF after storage. As in utero, fetus can absorb large volumes of AF but would infants with short gut syndrome or gastroschisis be able to tolerate enteral AF infusion which will prevent or decrease villous atrophy? Early trophic feeding in preterm infants is well established. Extremely low birth weight infants are not fed for the first few days. Would feeding AF during this time be beneficial to these infants? So there are many questions which are not well understood regarding how AF borne TF work together and lead to the development of a mature GIT.
FUTURE DIRECTIONS
Many questions about AF remain unanswered. The functions and significance of individual growth factors in human AF remain incompletely described[18]. There is also much to be learned about the immunoprotective properties of AF. Future directions include research into the use of synthetic or harvested AF as enteral infusions to promote the development of the GI tract in premature infants or infants recovering from NEC[18]. Which TF should be included in the simulated AF? The important question of whether TF would survive the process of storage and freezing is yet to be answered. Although many TF have been elicited and described in human AF, many remain to be understood completely and even more remain to be discovered. More in vivo studies are required with individual TF as well with a combination of different TF. At the same time, in vivo studies are required to see the role of individual growth factors in AF by blocking different TF. At present, AF remains a potential fluid with possible benefits to extremely premature infants until a definitive beneficial role is confirmed by more research and until its safety is proven.
Footnotes
Conflict-of-interest statement: All of the authors do not have any conflict of interest to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 30, 2015
First decision: September 28, 2015
Article in press: January 7, 2016
P- Reviewer: Caboclo JLF, Chow WK S- Editor: Kong JX L- Editor: A E- Editor: Jiao XK
References
- 1.Wagner CL, Taylor SN, Johnson D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clin Rev Allergy Immunol. 2008;34:191–204. doi: 10.1007/s12016-007-8032-3. [DOI] [PubMed] [Google Scholar]
- 2.Grand RJ, Watkins JB, Torti FM. Development of the human gastrointestinal tract. A review. Gastroenterology. 1976;70:790–810. [PubMed] [Google Scholar]
- 3.Klein RM, McKenzie JC. The role of cell renewal in the ontogeny of the intestine. I. Cell proliferation patterns in adult, fetal, and neonatal intestine. J Pediatr Gastroenterol Nutr. 1983;2:10–43. doi: 10.1097/00005176-198302010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Klein RM, McKenzie JC. The role of cell renewal in the ontogeny of the intestine. II. Regulation of cell proliferation in adult, fetal, and neonatal intestine. J Pediatr Gastroenterol Nutr. 1983;2:204–228. [PubMed] [Google Scholar]
- 5.Underwood MA, Sherman M. Nutritional Characteristics of Amniotic Fluid. NeoReviews. 2006;7:e310–e316. [Google Scholar]
- 6.Surana R, Puri P. Small intestinal atresia: effect on fetal nutrition. J Pediatr Surg. 1994;29:1250–1252. doi: 10.1016/0022-3468(94)90816-8. [DOI] [PubMed] [Google Scholar]
- 7.Hirai C, Ichiba H, Saito M, Shintaku H, Yamano T, Kusuda S. Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. J Pediatr Gastroenterol Nutr. 2002;34:524–528. doi: 10.1097/00005176-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Weaver LT, Walker WA. Epidermal growth factor and the developing human gut. Gastroenterology. 1988;94:845–847. doi: 10.1016/0016-5085(88)90263-6. [DOI] [PubMed] [Google Scholar]
- 9.Neu J, Li N. The neonatal gastrointestinal tract: developmental anatomy, physiology, and clinical implications. NeoReviews. 2003;4:e7–e13. [Google Scholar]
- 10.Godlewski MM, Słupecka M, Woliński J, Skrzypek T, Skrzypek H, Motyl T, Zabielski R. Into the unknown--the death pathways in the neonatal gut epithelium. J Physiol Pharmacol. 2005;56 Suppl 3:7–24. [PubMed] [Google Scholar]
- 11.Brandtzaeg P. The secretory immunoglobulin system: Regulation and biological significance. In: Davis M, Isaacs C (eds), editors. Integrating population outcomes, biological mechanisms and research methods in the study of human milk and lactation. Vol. 503. New York: Springer; 2002. pp. 1–16. [Google Scholar]
- 12.van Odijk J, Kull I, Borres MP, Brandtzaeg P, Edberg U, Hanson LA, Høst A, Kuitunen M, Olsen SF, Skerfving S, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966-2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy. 2003;58:833–843. doi: 10.1034/j.1398-9995.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 13.Sherman P, Forstner J, Roomi N, Khatri I, Forstner G. Mucin depletion in the intestine of malnourished rats. Am J Physiol. 1985;248:G418–G423. doi: 10.1152/ajpgi.1985.248.4.G418. [DOI] [PubMed] [Google Scholar]
- 14.Brace RA, Wolf EJ. Normal amniotic fluid volume changes throughout pregnancy. Am J Obstet Gynecol. 1989;161:382–388. doi: 10.1016/0002-9378(89)90527-9. [DOI] [PubMed] [Google Scholar]
- 15.Cummins AG, Thompson FM. Effect of breast milk and weaning on epithelial growth of the small intestine in humans. Gut. 2002;51:748–754. doi: 10.1136/gut.51.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulvihill SJ, Stone MM, Debas HT, Fonkalsrud EW. The role of amniotic fluid in fetal nutrition. J Pediatr Surg. 1985;20:668–672. doi: 10.1016/s0022-3468(85)80021-x. [DOI] [PubMed] [Google Scholar]
- 17.Wintour EM, Shandley L. Effects of fetal fluid balance on amniotic fluid volume. Semin Perinatol. 1993;17:158–172. [PubMed] [Google Scholar]
- 18.Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25:341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert WM, Brace RA. Amniotic fluid volume and normal flows to and from the amniotic cavity. Semin Perinatol. 1993;17:150–157. [PubMed] [Google Scholar]
- 20.Gilbert WM, Newman PS, Eby-Wilkens E, Brace RA. Technetium Tc 99m rapidly crosses the ovine placenta and intramembranous pathway. Am J Obstet Gynecol. 1996;175:1557–1562. doi: 10.1016/s0002-9378(96)70106-0. [DOI] [PubMed] [Google Scholar]
- 21.Brace RA. Physiology of amniotic fluid volume regulation. Clin Obstet Gynecol. 1997;40:280–289. doi: 10.1097/00003081-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Chochinov RH, Ketupanya A, Mariz IK, Underwood LE, Daughaday WH. Amniotic fluid reactivity detected by somatomedin C radioreceptor assay: correlation with growth hormone, prolactin and fetal renal maturation. J Clin Endocrinol Metab. 1976;42:983–986. doi: 10.1210/jcem-42-5-983. [DOI] [PubMed] [Google Scholar]
- 23.Barka T, van der Noen H, Gresik EW, Kerenyi T. Immunoreactive epidermal growth factor in human amniotic fluid. Mt Sinai J Med. 1978;45:679–684. [PubMed] [Google Scholar]
- 24.Maheshwari A. Role of cytokines in human intestinal villous development. Clin Perinatol. 2004;31:143–155. doi: 10.1016/j.clp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Wagner CL, Forsythe DW. Effect of human milk and recombinant EGF, TGFalpha, and IGF-1 on small intestinal cell proliferation. Adv Exp Med Biol. 2000;478:373–374. doi: 10.1007/0-306-46830-1_33. [DOI] [PubMed] [Google Scholar]
- 26.Booth C, Evans GS, Potten CS. Growth factor regulation of proliferation in primary cultures of small intestinal epithelium. In Vitro Cell Dev Biol Anim. 1995;31:234–243. doi: 10.1007/BF02639439. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555–1562. [PubMed] [Google Scholar]
- 28.Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992;102:695–698. doi: 10.1016/0016-5085(92)90123-g. [DOI] [PubMed] [Google Scholar]
- 29.Playford RJ, Wright NA. Why is epidermal growth factor present in the gut lumen? Gut. 1996;38:303–305. doi: 10.1136/gut.38.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacy ER. Epithelial restitution in the gastrointestinal tract. J Clin Gastroenterol. 1988;10 Suppl 1:S72–S77. doi: 10.1097/00004836-198812001-00012. [DOI] [PubMed] [Google Scholar]
- 31.Playford RJ, Marchbank T, Calnan DP, Calam J, Royston P, Batten JJ, Hansen HF. Epidermal growth factor is digested to smaller, less active forms in acidic gastric juice. Gastroenterology. 1995;108:92–101. doi: 10.1016/0016-5085(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 32.Varner MW, Dildy GA, Hunter C, Dudley DJ, Clark SL, Mitchell MD. Amniotic fluid epidermal growth factor levels in normal and abnormal pregnancies. J Soc Gynecol Investig. 1996;3:17–19. doi: 10.1016/1071-5576(95)00044-5. [DOI] [PubMed] [Google Scholar]
- 33.Tremblay E, Monfils S, Ménard D. Epidermal growth factor influences cell proliferation, glycoproteins, and lipase activity in human fetal stomach. Gastroenterology. 1997;112:1188–1196. doi: 10.1016/s0016-5085(97)70130-6. [DOI] [PubMed] [Google Scholar]
- 34.Claud EC, Savidage T, Walker WA. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr Res. 2003;53:419–425. doi: 10.1203/01.PDR.0000050141.73528.AD. [DOI] [PubMed] [Google Scholar]
- 35.Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J, Athar M, Shimamura M, Bhandari V, Aprahamian C, et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology. 2011;140:242–253. doi: 10.1053/j.gastro.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimble RM, Breier BH, Gluckman PD, Harding JE. Enteral IGF-I enhances fetal growth and gastrointestinal development in oesophageal ligated fetal sheep. J Endocrinol. 1999;162:227–235. doi: 10.1677/joe.0.1620227. [DOI] [PubMed] [Google Scholar]
- 37.Merimee TJ, Grant M, Tyson JE. Insulin-like growth factors in amniotic fluid. J Clin Endocrinol Metab. 1984;59:752–755. doi: 10.1210/jcem-59-4-752. [DOI] [PubMed] [Google Scholar]
- 38.Lallemand AV, Ruocco SM, Joly PM, Gaillard DA. In vivo localization of the insulin-like growth factors I and II (IGF I and IGF II) gene expression during human lung development. Int J Dev Biol. 1995;39:529–537. [PubMed] [Google Scholar]
- 39.Constância M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 40.Semba RD, Juul SE. Erythropoietin in human milk: physiology and role in infant health. J Hum Lact. 2002;18:252–261. doi: 10.1177/089033440201800307. [DOI] [PubMed] [Google Scholar]
- 41.Calhoun DA, Lunoe M, Du Y, Christensen RD. Granulocyte colony-stimulating factor is present in human milk and its receptor is present in human fetal intestine. Pediatrics. 2000;105:e7. doi: 10.1542/peds.105.1.e7. [DOI] [PubMed] [Google Scholar]
- 42.Barmeyer C, Horak I, Zeitz M, Fromm M, Schulzke JD. The interleukin-2-deficient mouse model. Pathobiology. 2003;70:139–142. doi: 10.1159/000068145. [DOI] [PubMed] [Google Scholar]
- 43.Rollwagen FM, Yu ZY, Li YY, Pacheco ND. IL-6 rescues enterocytes from hemorrhage induced apoptosis in vivo and in vitro by a bcl-2 mediated mechanism. Clin Immunol Immunopathol. 1998;89:205–213. doi: 10.1006/clin.1998.4600. [DOI] [PubMed] [Google Scholar]
- 44.Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr. 1999;23:S3–S6. doi: 10.1177/014860719902300502. [DOI] [PubMed] [Google Scholar]
- 45.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 2001;15:1398–1403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 46.Altman DJ, Schneider SL, Thompson DA, Cheng HL, Tomasi TB. A transforming growth factor beta 2 (TGF-beta 2)-like immunosuppressive factor in amniotic fluid and localization of TGF-beta 2 mRNA in the pregnant uterus. J Exp Med. 1990;172:1391–1401. doi: 10.1084/jem.172.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, Neal MD, Yazji I, Jia H, Lin J, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci USA. 2012;109:11330–11335. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siggers J, Ostergaard MV, Siggers RH, Skovgaard K, Mølbak L, Thymann T, Schmidt M, Møller HK, Purup S, Fink LN, et al. Postnatal amniotic fluid intake reduces gut inflammatory responses and necrotizing enterocolitis in preterm neonates. Am J Physiol Gastrointest Liver Physiol. 2013;304:G864–G875. doi: 10.1152/ajpgi.00278.2012. [DOI] [PubMed] [Google Scholar]
- 49.Zani A, Cananzi M, Fascetti-Leon F, Lauriti G, Smith VV, Bollini S, Ghionzoli M, D’Arrigo A, Pozzobon M, Piccoli M, et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut. 2014;63:300–309. doi: 10.1136/gutjnl-2012-303735. [DOI] [PubMed] [Google Scholar]
- 50.Jain SK, Baggerman EW, Mohankumar K, Namachivayam K, Jagadeeswaran R, Reyes VE, Maheshwari A. Amniotic fluid-borne hepatocyte growth factor protects rat pups against experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G361–G369. doi: 10.1152/ajpgi.00272.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golovin DI, Nikonov AA. APUD cells and apudomas. Arkh Patol. 1981;43:3–11. [PubMed] [Google Scholar]
- 52.Bosman FT, Louwerens JW. APUD cells in teratomas. Am J Pathol. 1981;104:174–180. [PMC free article] [PubMed] [Google Scholar]
- 53.Andrew A. An experimental investigation into the possible neural crest origin of pancreatic APUD (islet) cells. J Embryol Exp Morphol. 1976;35:577–593. [PubMed] [Google Scholar]
- 54.Petsche Connell J, Camci-Unal G, Khademhosseini A, Jacot JG. Amniotic fluid-derived stem cells for cardiovascular tissue engineering applications. Tissue Eng Part B Rev. 2013;19:368–379. doi: 10.1089/ten.teb.2012.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Playford RJ. Peptides and gastrointestinal mucosal integrity. Gut. 1995;37:595–597. doi: 10.1136/gut.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seare NJ, Playford RJ. Growth factors and gut function. Proc Nutr Soc. 1998;57:403–408. doi: 10.1079/pns19980057. [DOI] [PubMed] [Google Scholar]
- 57.Balda MS, Fallon MB, Van Itallie CM, Anderson JM. Structure, regulation, and pathophysiology of tight junctions in the gastrointestinal tract. Yale J Biol Med. 1992;65:725–735; discussion 737-740. [PMC free article] [PubMed] [Google Scholar]
- 58.Axelsson I, Jakobsson I, Lindberg T, Polberger S, Benediktsson B, Räihä N. Macromolecular absorption in preterm and term infants. Acta Paediatr Scand. 1989;78:532–537. doi: 10.1111/j.1651-2227.1989.tb17932.x. [DOI] [PubMed] [Google Scholar]
- 59.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 60.Sullivan SE, Calhoun DA, Maheshwari A, Ashmeade TL, Auerbach DA, Hudak ML, Beltz SE, Christensen RD. Tolerance of simulated amniotic fluid in premature neonates. Ann Pharmacother. 2002;36:1518–1524. doi: 10.1345/aph.1A439. [DOI] [PubMed] [Google Scholar]
- 61.Lima-Rogel V, Calhoun DA, Maheshwari A, Torres-Montes A, Roque-Sanchez R, Garcia MG, Christensen RD. Tolerance of a sterile isotonic electrolyte solution containing select recombinant growth factors in neonates recovering from necrotizing enterocolitis. J Perinatol. 2003;23:200–204. doi: 10.1038/sj.jp.7210894. [DOI] [PubMed] [Google Scholar]
- 62.Porter AE, Auth J, Prince M, Ghidini A, Brenneman DE, Spong CY. Optimization of cytokine stability in stored amniotic fluid. Am J Obstet Gynecol. 2001;185:459–462. doi: 10.1067/mob.2001.115106. [DOI] [PubMed] [Google Scholar]


