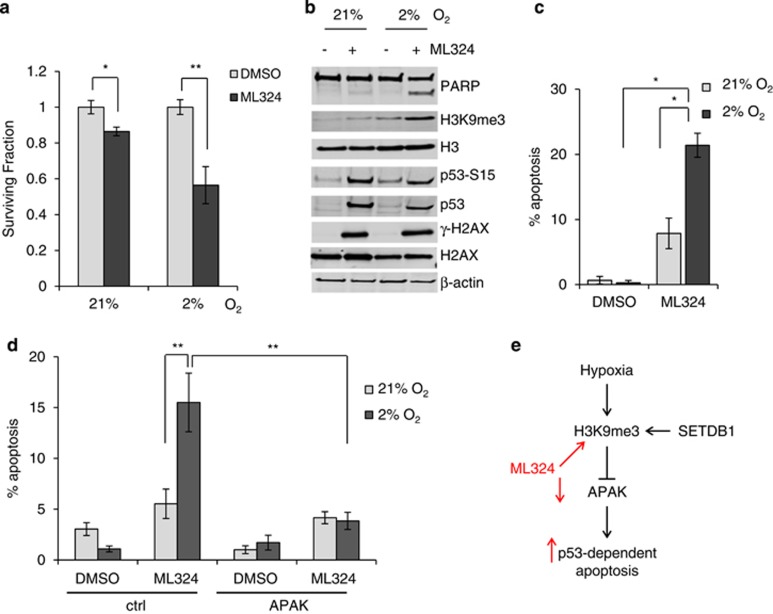
Figure 4.
Pharmacological manipulation of H3K9me3 levels affects cell viability and apoptosis in hypoxia. (a) RKO cells were treated with 1 μM JMJD2 (JMJD2E) inhibitor, ML324 (Axon Medchem, Groningen, The Netherlands) or DMSO and exposed to 21 or 2% O2 for 24 h. Clonogenic survival assays were carried out. Colonies (of at least 50 cells) were allowed to form for 7–10 days. Colonies were then stained with methylene blue and counted. Error bars indicate standard error between technical replicates. A representative graph of one of three independent experiments is shown for all figures showing clonogenic assays unless otherwise stated. (b) RKO cells were treated with either DMSO or ML324 (10 μM) and exposed to either 21 or 2% O2 for 48 h. Western blotting was carried out with the antibodies indicated as previously described. H2A (Calbiochem, Billerica, MA, USA), γH2AX (Upstate/Millipore) and PARP (Cell Signaling). (c) RKO cells were treated as in (b). Apoptosis by nuclear morphology was measured as the number of apoptotic/nonapoptotic cells expressed as a percentage of the whole population. (d) RKO cells transfected with either Myc-APAK or Myc-empty plasmids and treated with 10 μM ML324 for 24 h followed by incubation in 21% or 2% O2 for an additional 24 h. Apoptosis was measured as in (c). (e) Schematic representation of the proposed model. Hypoxia increases H3K9me3 levels, which in turn leads to APAK repression and p53-dependent apoptosis. Increased H3K9me3 levels and subsequent decreased APAK expression results from both pharmacological inhibition of JMJD2 enzymes (ML324) or through the action of methyltransferase SETDB1.