Figure 3.
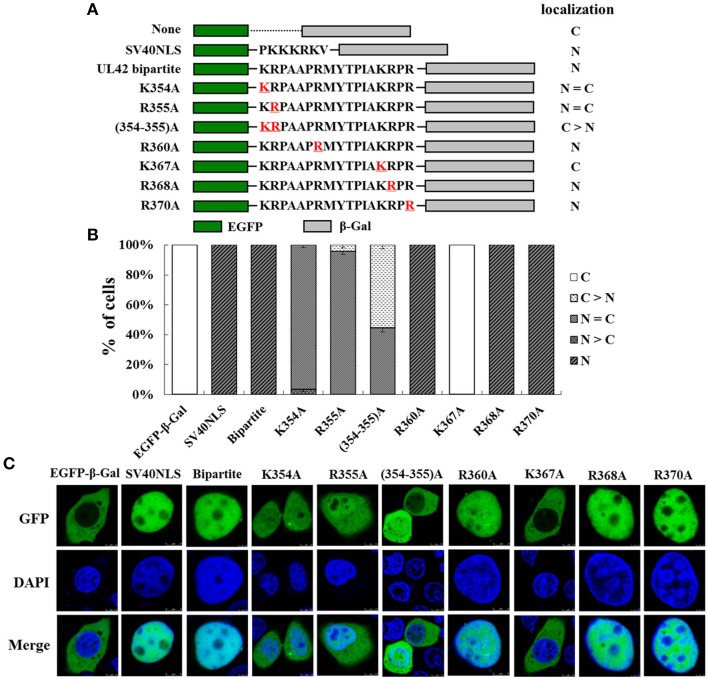
K354, R355, and K367 are important for the function of the UL42 bipartite NLS. (A) Schematic representation of the classical SV40 TAg-NLS, the UL42 bipartite NLS, and the UL42 NLS bearing amino acid substitutions fused between EGFP and β-Gal. “None” indicates that no specific motif was fused between these two reporter proteins. The red underlined amino acids were changed to A residue at the indicated positions. The localization of these constructs is summarized on the right. N, exclusively nuclear; N = C, diffuse; C > N, more cytoplasmic than nuclear; C, strictly cytoplasmic. (B) For each construct, 100 EGFP-expressing cells were scored after independent transfections in three repeated experiments and the relative percentages of the different subcellular localizations of the fusion constructs were estimated. N, exclusively nuclear; N > C, more nuclear than cytoplasmic; N = C, diffuse; C > N, more cytoplasmic than nuclear; C, strictly cytoplasmic. (C) Subcellular localization of various EGFP–β-Gal fusion proteins containing the specific NLS was analyzed with confocal microscopic analysis of the fluorescent EGFP signal and the Hoechst-reagent-stained DNA. The merged GFP and DAPI signals are shown. The image for each construct is representative of three independent transfection experiments.