Figure 4. Real time NMR signal intensity change accompanied by the peptide bond cleavage at L-α- and D-β-Asp58 of αA-crystallin 51–60 fragment.
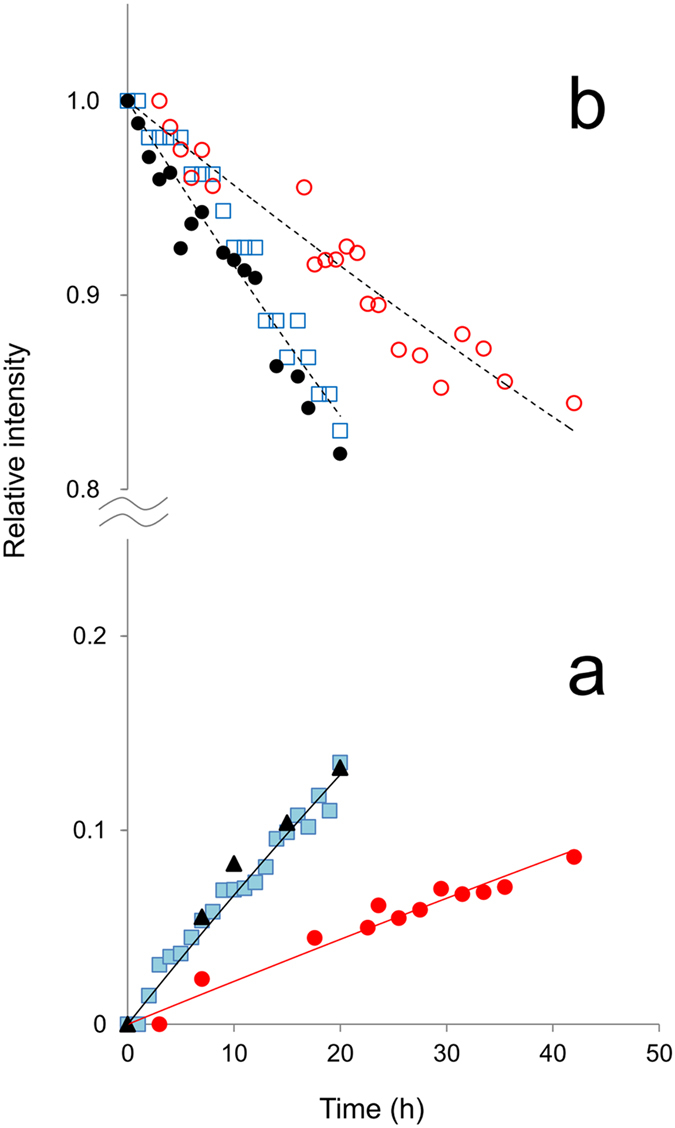
The increase in the products C-terminal L-Asp58 ( ) of 51–58 fragment and N-terminal Ser59 of Ser59-Gly60 (
) of 51–58 fragment and N-terminal Ser59 of Ser59-Gly60 ( ) from the reactant L-α-Asp 51–60 is plotted in (a), together with the increase in N-terminal Ser59 (
) from the reactant L-α-Asp 51–60 is plotted in (a), together with the increase in N-terminal Ser59 ( ) from the reactant D-β-Asp 51–60. Each symbol designates integral intensity of the respective signal. In (b), the decrease of the reactant non-terminal L-α-Asp58 evaluated from integral intensities (
) from the reactant D-β-Asp 51–60. Each symbol designates integral intensity of the respective signal. In (b), the decrease of the reactant non-terminal L-α-Asp58 evaluated from integral intensities ( ) and peak heights (
) and peak heights ( ) is shown, as well as that of non-terminal D-β-Asp58 estimated from peak heights (
) is shown, as well as that of non-terminal D-β-Asp58 estimated from peak heights ( ). All values are relative to the initial intensities. Black and red solid lines in (a) are the resulting curves obtained by fitting equation 2 to the respective experimental values, from which the rate constants k were estimated. Dashed lines in (b) are only guide for eyes.
). All values are relative to the initial intensities. Black and red solid lines in (a) are the resulting curves obtained by fitting equation 2 to the respective experimental values, from which the rate constants k were estimated. Dashed lines in (b) are only guide for eyes.
