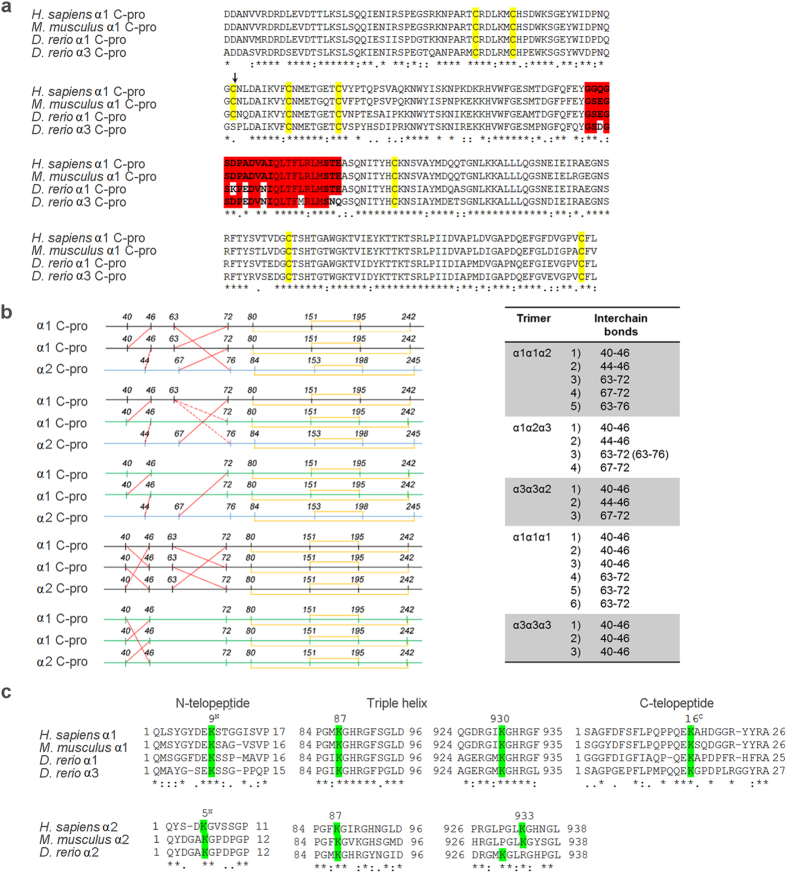
Figure 2. Comparison of conserved protein regions and amino acids with crucial function in type I procollagen chain assembly and extracellular collagen fibril formation.
(a) Alignment of the α1 and α3 C-propeptide sequence in D. rerio with the α1 C-propeptide of human (H. sapiens) and mouse (M. musculus). In the recognition sequence (highlighted in red), responsible for the α chain assembly in a trimeric molecule, the α3 chain has 6 different AA when compared to human/mouse α1 whereas for α1 only 3 AA are different. The cysteine residues responsible for inter- and intra-chain disulfide bonds (yellow) are all conserved in zebrafish α1 but Cys63 (arrow), involved in inter-chain bonds, is missing in α3 and substituted with a Ser. (b) Representative pattern of the inter- and intra-chain disulfide bonds (in red and yellow respectively) in all the possible trimer compositions. (c) Lys/Hyl residues known to be involved in type I collagen cross-link stabilization are highlighted in green. The human/mouse Lys/Hyl α2(I) 933 is substituted in zebrafish α2 by an arginine, but a Lys/Hyl is present in position 930.