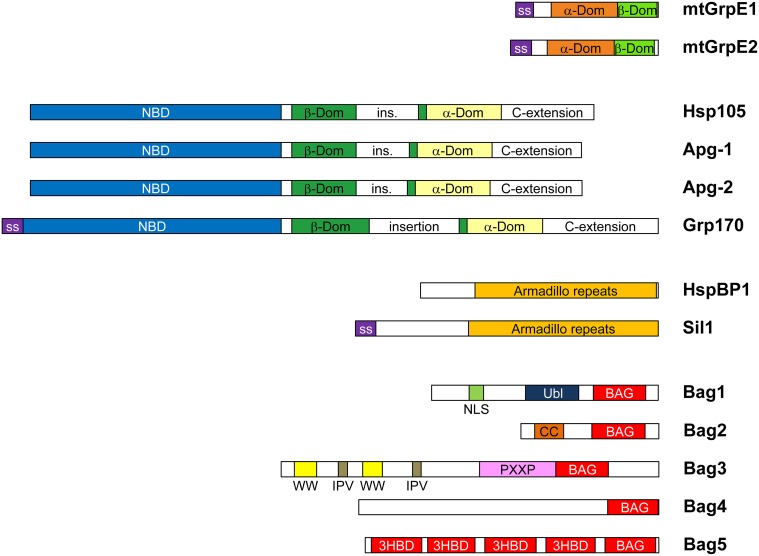
Figure 1.
Domain compositions of human NEF homologs. Human cells express two mitochondrial GrpE isoforms (mtGrpE1/GRPEL1 and mtGrpE2/GRPEL2), three Hsp110 homologs (Hsp105/HSPH1, Apg-1/HSPA4L, and Apg-2/HSPA4—the codes designate the gene names) and five BAG-domain proteins. There is only one form of Grp170/HYOU1, HspBP1, and Sil1, respectively. Isoforms arising from alternative initiation sites and splicing were described for Hsp105, HspBP1, and Bag1 (not shown). mtGrpE isoforms contain mitochondrial signal sequences (ss). The α- and β-domains (orange and green, respectively) are conserved with GrpE from E. coli. The Hsp110/Grp170 family proteins consist of an N-terminal nucleotide binding domain (NBD, blue), a β-sandwich (β-Dom, green) and a α-helix bundle domain (α-Dom, pale yellow). All isoforms contain long variable insertions in the β-sandwich and at the C-terminus. SS indicates signal sequences for ER import of Grp170 and Sil1. HspBP1 and Sil have characteristic Armadillo repeat folds (orange). All members of the BAG family in humans, Bag1-5, contain C-terminal Hsp70-binding BAG domains (red), but have otherwise divergent domain composition. Bag1 contains an Ubiquitin-like domain (Ubl, dark blue), which might associate with the regulatory particle of the 26S proteasome, and a NLS sequences (purple) for nuclear targeting. Bag2 contains a coiled-coil dimerization domain (CC, orange) (Page et al., 2012). Bag3 comprises multiple N-terminal sequence motifs including WW domains (WW, yellow), IPV sequence motifs (brown) and PXXP repeats (pink), which mediate interactions with proline-rich motifs, HspB8 and SH3 domains, respectively (Doong et al., 2000; Fuchs et al., 2010; Iwasaki et al., 2010; Ulbricht et al., 2013). Bag5 has four additional 3-helix bundle domains of unknown function (Arakawa et al., 2010). Bag6 is not shown because the original assignment as an NEF of Hsp70 was incorrect (Mock et al., 2015).