Abstract
Background:
The fruits of Barringtonia racemosa and Hibiscus sabdariffa have been used in the treatment of abscess, ulcer, cough, asthma, and diarrhea as traditional remedy.
Objective:
This study aims to evaluate cytotoxic effect of B. racemosa and H. sabdariffa methanol fruit extracts toward human breast cancer cell lines (MCF-7) and its antioxidant activities.
Materials and Methods:
Total antioxidant activities of extracts were assayed using 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH) and β-carotene bleaching assay. Content of phytochemicals, total flavonoid content (TFC), and total phenolic content (TPC) were determined using aluminum chloride colorimetric method and Folin–Ciocalteu's reagent, respectively. Cytotoxic activity in vitro was investigated through 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay.
Results:
B. racemosa extract exhibited high antioxidant activities compared to H. sabdariffa methanol fruit extracts in DPPH radical scavenging assay (inhibitory concentration [IC50] 15.26 ± 1.25 μg/mL) and ί-carotene bleaching assay (I% 98.13 ± 1.83%). B. racemosa also showed higher TPC (14.70 ± 1.05 mg gallic acid equivalents [GAE]/g) and TFC (130 ± 1.18 mg quercetin equivalents [QE]/g) compared to H. sabdariffa (3.80 ± 2.13 mg GAE/g and 40.75 ± 1.15 mg QE/g, respectively). In MTT assay, B. racemosa extract also showed a higher cytotoxic activity (IC50 57.61 ± 2.24 μg/mL) compared to H. sabdariffa.
Conclusion:
The present study indicated that phenolic and flavonoid compounds known for oxidizing activities indicated an important role among the contents of these plants extract. B. racemosa methanol extract have shown potent cytotoxic activity toward MCF-7. Following these promising results, further fractionation of the plant extract is underway to identify important phytochemical bioactives for the development of potential nutraceutical and pharmaceutical use.
SUMMARY
The phenolic and flavonoid compounds were present in B. racemosa and H. sabdariffa methanol extracts
B. racemosa methanol extract was found to be potent antioxidant activity
B. racemosa methanol extract have shown potent cytotoxic activity (IC50 57.61 ± 2.24 μg/mL) toward MCF-7
The phenolic and flavonoid compounds may contribute to the antioxidant and cytotoxic activity of B. racemosa.
Abbreviations Used: MCF-7: Human breast cancer cell lines, DMEM: Modified eagle medium, DPPH: 2,2’-diphenyl-1-picrylhydrazyl radical, TPC: Total phenolic content, Na2CO3: Sodium carbonate, GAE: Gallic acid equivalents, TFC: Total flavonoid content, NaNO2: Sodium nitrite, AlCl3: Aluminum chloride, NaOH: Sodium hydroxide, QE: Quercetin equivalents, MTT: 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide, IC50: Inhibitory concentration, ANOVA: Analysis of variance, DLA: Dalton's lymphoma ascitic.
Keywords: Antioxidant activities, Barringtonia racemosa, Cytotoxic activity, Hibiscus sabdariffa, Phytochemistry
INTRODUCTION
In recent decades, natural antioxidants have been garnering more attention due to the less adverse reactions compared to synthetic agents. Antioxidants have the ability to prevent some cell damage caused by free radicals and reactive oxygen species. Antioxidant activity as a research methodology is important to assess the scientific basis for the known traditional herbal medicines which claimed to have antioxidant properties.[1,2]
Cancer is a much-feared disease in the modern society, and is one of the leading causes of mortality worldwide.[3] The National Cancer Registry of Malaysia reported a total of 21,773 cancer cases diagnosed in Peninsular Malaysia in 2006.[4] An ideal anticancer agent is expected to inhibit the progression of cancer through its cytotoxicity properties.[5] Identification of cytotoxic compounds contributed to the development of anticancer therapeutics. Plant-based traditional medicine has played a major role in the therapy of a spectrum of diseases including cancer, and most of the researchers are giving attention to natural products.[6] To date, natural products are the most prolific source of biologically active compounds and play important role in the drug discovery and development.
Barringtonia racemosa, locally known as putat sungai in Malaysia, is a rich source of phytomedicine. In this region, the fruit and seed are used to treat asthma, cough, abscess, ulcer, and diarrhea.[7] Quantitative phytochemical analysis of ethanolic extracts of B. racemosa aerial parts have showed higher content of total flavonoids.[8] Hibiscus sabdariffa known as roselle is an annual shrub commonly used to make jellies, jams, and beverages. The dried flower of H. sabdariffa extract has shown high levels of polyphenol, flavonoid, and anthocyanin which was observed to be associated with anticancer effects.[9]
Despite a few isolation works done on selected part of the plants, data on antioxidant potential of fruit part of the B. racemosa are still lacking. Information on antioxidant and cytotoxic activity of the plant extract worked potentially shed light on mechanism related to bioactive(s) of the plant. In this study, a Malaysian local tropical plant B. racemosa had been selected to identify for its antioxidant and anticancer activity as well as to compare the antioxidant capacity and cytotoxicity effect with a selected top source of antioxidant of local plant, H. sabdariffa.
MATERIALS AND METHODS
Chemicals
2,2′- diphenyl-1-picrylhydrazyl radical (DPPH), Folin–Ciocalteu phenol reagent, sodium carbonate (Na2 CO3), β-carotene, linoleic acid, Tween 40, sodium nitrite (NaNO2), aluminum chloride (AlCl3), sodium hydroxide, ascorbic acid, gallic acid, and quercetin were obtained from Sigma-Aldrich (Sigma Chemical Co., St Louis, MO, USA). All the chemicals were of analytical grade.
Preparation of plant extracts
The whole fruits of B. racemosa and H. sabdariffa were collected from Permatang Pauh, Pulau Pinang, Malaysia. The fruits were cleaned and separated from the seed. The fruits were dried in an oven, at an average temperature of 40°C. Then, the fruits were grounded into powders. Dried powder of B. racemosa and H. sabdariffa fruits, respectively, was macerated with methanol at a ratio of dry weight:solvent of 1:10 (w/v). The extracts were filtered and evaporated on rotary evaporator to yield a crude residue. The crude extracts were stored in refrigerator and were evaluated for total phenolic content (TPC), total flavonoid content (TFC) as well as antioxidants activity.
Determination of total phenolic content
The TPC was measured using Folin–Ciocalteu's reagent according to the literature with some modifications.[10] Briefly, 0.1 mL extracts (1 mg/mL) was mixed with 1.0 mL Foline–Ciocalteu phenol reagent and allowed to react for 3 min. Then, 300 mL of 1N Na2CO3 was added and incubated at room temperature for 90 min. Gallic acid (0.02–0.64 mg/mL) was used as standard. Absorbance was measured at 725 nm using Thermo Scientific™ Multiskan™ GO Microplate spectrophotometer. TPC of the extracts were determined from the standard calibration graph of absorbance versus concentration. The results were expressed as mg gallic acid equivalents (GAE) in 1 g of sample (mg GAE/g).
Determination of total flavonoid content
The TFC of the extracts was measured using colorimetric method with some modifications based on the stable formation of a flavonoid aluminum complex.[11] Briefly, 250 mL of extracts (1 mg/mL) were mixed with 1.25 mL of distilled water and 75 mL of 5% NaNO2 solution. After 6 min, 150 mL of 10% AlCl3 was added, and the mixture was allowed to stand for a further 5 min. Then, 0.5 mL of 1 M sodium hydroxide (NaOH) and 275 mL distilled water were added to the mixture. Quercetin (0.02–0.64 mg/mL) was used as standard. Absorbance was measured at λmax 510 nm using a Thermo Scientific™ Multiskan™ GO Microplate spectrophotometer. TFCs of the extracts were determined from the standard calibration graph of absorbance versus concentration. The results were expressed as mg quercetin equivalents in 1 g of sample (mg quercetin equivalents [QE]/g).
2,2′- diphenyl-1-picrylhydrazyl radical radical scavenging assay
The free radical scavenging activity was evaluated to look at the oxidation ability of the plant extracts toward the free radical DPPH. Experiments were carried out according to the literature with some modifications.[12] Briefly, 200 μL of extract solution with different concentrations (1.953–1000 μg/mL) was loaded in 96-well plate. DPPH in absolute methanol (50 μL, 1 mM) was added into wells. The reaction mixture was shaken well and incubated for 30 min at room temperature in the dark. The absorbance of the resulting solution was recorded at 517 nm. The test was performed in triplicate. The inhibition percentage (I%) of DPPH was calculated according to the following equation:
IC (%) = (A0 − At)/A0 × 100
Where, IC (%) is a I% of DPPH radical. A0 is the absorbance of a blank that was prepared in the same conditions, but without sample, and At is the absorbance of test samples. Ascorbic acid was used as a positive control. A lower inhibitory concentration (IC50) value indicates greater antioxidant activity.
β-carotene-linoleic acid bleaching assay
The antioxidant activities of the extracts were determined using the β-carotene-linoleic acid bleaching assay according to the literature with some modifications.[13,14] A stock solution of β-carotene-linoleic acid was prepared by mixing 1 mL of chloroform with 0.5 mg β-carotene, 25 μL linoleic acid, and 200 mg Tween 40 into round bottom flask. The mixture was then evaporated at 40°C for 10 min by rotary evaporator to remove chloroform and immediately diluted with 100 mL of distilled water. The water was added to the mixture with vigorous shaking to form an emulsion. Then, 2.5 mL emulsion and 350 μL extracts (1 mg/mL) were added. The system was incubated in hot water bath at 45°C for 1 h. The absorbance of the samples, standard, and control was measured at 470 nm using a Thermo Scientific™ Multiskan™ GO Microplate spectrophotometer against a blank, consisting of an emulsion without β-carotene. The measurement was carried out at initial time (t = 0) and successively at 15 min intervals for 120 min. All samples were assayed in triplicate. I% of the samples was calculated using following equation:
I (%) = (A1/A0) ×100
Where, A1 is the absorbance of β-carotene after 1 h assay remaining in the samples and A0 is the absorbance of β-carotene at beginning of the experiments. Ascorbic acid was used as a positive control. A higher I% value indicates greater antioxidant activity.
Anticancer activity assay
Cell culture
The human breast cancer cell lines (MCF-7) were maintained in Dulbecco's modified eagle medium supplemented with 10% heat inactivated fetal bovine serum, streptomycin (100 μg/mL), and penicillin (100 μg/mL) in culture flask at 37°C in 5% CO2 incubator. Subculture will be performed every 3 days.
Cytotoxic activity by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide assay
The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay on crude extracts was carried out according to the method developed by[15] with slight modifications using MCF-7. The carcinoma cells were seeded in a 96-well plate and incubated for 24 h. After 24 h, 100 μl of medium containing extracts with various concentrations (200 μg/mL, 100 μg/mL, 50 μg/mL, 25 μg/mL, 12.5 μg/mL, and 6.25 μg/mL) were added into 96-well plates and further incubated for 48 h. A positive control (tamoxifen), blank (medium only), and negative control (cell with medium only) were included. Then, 24 μl of 5 mg/mL MTT reagent was added to each well and incubated for another 4 h. After that, medium was aspirated and 100 μl of DMSO was added to each well. After 10 min incubation, the absorbance was measured at 570 nm using a Thermo Scientific™ Multiskan™ GO Microplate spectrophotometer. I%s of the cells were calculated using following equation.
IC (%) = (1 - [At -A0]/[A1 − A0]) × 100
Where, (IC) (%) is a I% of the cells. At is the absorbance of test samples, A0 is the absorbance of a blank and A1 is the absorbance of a negative control. Tamoxifen was used as a positive control. A lower IC50 value indicates greater anticancer activity.
Statistical analysis
All experiments were carried out in triplicates and results are expressed as means ± standard error mean. Data were analyzed using one-way analysis of variance and differences were considered significant at P < 0.05. The ICs50 were determined by statistical software, GraphPad Software, Inc. Version 5.00.
RESULTS
Determination of total phenolic and total flavonoid content
Phytochemical screening of total phenolic and TFC revealed the presence of phenolic and flavonoid compounds in B. racemosa and H. sabdariffa methanol fruit extracts. The results of total phenolic and TFC assay are as shown in Table 1. In the assay, B. racemosa demonstrated highest TPC (14.70 ± 1.05 mg GAE/g) and TFC (130 ± 1.18 mg QE/g) compared to H. sabdariffa (3.80 ± 2.13 mg GAE/g and 40.75 ± 1.15 mg QE/g), respectively.
Table 1.
Phenolic and flavonoid contents of methanol fruit extracts of B. racemosa and H. sabdariffa

2,2′- diphenyl-1-picrylhydrazyl radical radical scavenging assay and β-carotene-linoleic acid bleaching assay
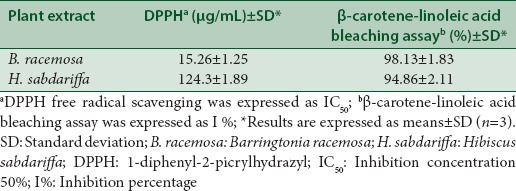
The methanol fruit extracts of B. racemosa and H. sabdariffa were tested for their antioxidant activity using DPPH and β-carotene-linoleic acid bleaching assay [Table 2]. The B. racemosa fruit extract was found to possess higher DPPH free radical scavenging activity at lowest concentration (IC50 15.26 ± 1.25 μg/mL) compared to H. sabdariffa (IC50 124.3 ± 1.89 μg/mL). This can be seen by determining the inhibition concentration (IC50) values of these two extract compared with standard ascorbic acid (7.86 ± 1.87 μg/mL). In β-carotene-linoleic acid bleaching assay, the highest percentage of inhibition was shown by B. racemosa fruit extract at 98.13 ± 1.83% followed by H. sabdariffa at 94.86 ± 2.11%.
Table 2.
Antioxidant activity of methanol fruit extracts of B. racemosa and H. sabdariffa assessed by DPPH and β-carotene-linoleic acid bleaching assay
Anticancer activity assay
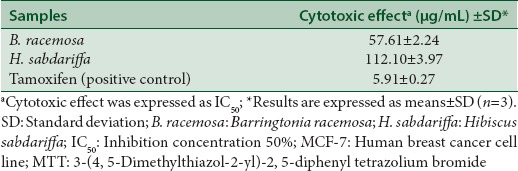
The cytotoxic effects of crude methanol extract of B. racemosa and H. sabdariffa on the growth of breast cancer cell line (MCF-7), as well as tamoxifen as positive control were investigated using the MTT assay and summarized in Table 3. Interestingly, the in vitro screening of B. racemosa fruit methanol extract showed potential cytotoxic activity against MCF-7 cell line compared to H. sabdariffa. The IC50 value obtained for B. racemosa was 42.63 μg/mL. This can be seen by determining the IC50 values of these two extracts compared with standard ascorbic acid (7.86 μg/mL).
Table 3.
Cytotoxic activity of methanol fruit extracts of B. racemosa and H. sabdariffa respectively on MCF-7 assessed by MTT assay
DISCUSSION
B. racemosa fruit has been consumed in Malaysia villages as traditional salad and it is interesting to discover that the flavonoid content was higher at almost 10 times compared to its phenolic content. This study concurred with previous finding which found phenolic and flavonoids as the major antioxidant components of the B. racemosa leaf, stick, and bark extracts.[8] H. sabdariffa methanol fruit extract in this study had showed a slightly high value of TPC compared with previous study on its methanol extract of seed, fruit, leaf, and stem methanol extract of the same species.[16] The TFC values also showed higher flavonoid content from a previous similar study.[17] The slight differences may be due to the geographical areas, state of maturity of sample sources, and method of sample preparation.[18]
The observed result in Table 2 reveal that B. racemosa possess antioxidant properties, since the extract showed potent low IC50 value (15.26 ± 1.25 μg/mL) in DPPH assay and high percentage of inhibition (98.13 ± 1.83%) in β-carotene linoleic acid bleaching assay. This activity could be due to the presence of phenolic and flavonoid compounds, as found by previous studies done on aerial part of the plant.[8] Of interest, the quercetin 3-O-rutinoside is known to be present in the methanol extract of B. racemosa fruit isolation work.[19] Luo et al. in 2002 have established how the contribution of total antioxidant activity of many fruits and vegetables was related to their flavonoid and polyphenols contents.[20] Several other reports have also demonstrated a close relationship between total phenolic and flavonoid content and high antioxidant activity.[21,22] This present study exposed that fruit of B. racemosa could be a good source of natural antioxidants due to it being rich in flavonoid and phenolic content. B. racemosa can be an interesting source of antioxidant bioactive, with potential uses in many industries such as food, cosmetics, nutraceutical, and pharmaceutical. These quantitative analyses of antioxidant activity are in support of the relationship between total antioxidant capacity and phytochemical content of plant extract of this species.
Consequently, in the last few years, the identification and development of natural product-based drug has become a major area in cancer research. In fact, approximately, 74% of anticancer drugs developed today originated from medicinal plants.[23] The fruit of B. racemosa are studied for the first time for their cytotoxic activities except for H. sabdariffa. Interestingly, in this study, a local plant B. racemosa fruit methanol extract showed potential cytotoxic activity (IC50 57.61 ± 2.24 μg/mL) against MCF-7 cell line. The cytotoxic activity in MCF-7 cell line may be due to the presence of phytochemicals content in extract. Several reports indicated that flavonoid contributed to programed cell death mechanism by activating caspases pathway.[24] The combination of both alkaloids with flavonoids in ononis hirta (aerial parts) extract was also has shown superior activity against MCF-7 cell line.[25] This result of our observed cytotoxicity is in accordance with the phytochemical finding which showed the presence of flavonoids and alkaloid in plant extracts [Table 1]. This study was comparable to the previous finding that was found on the seed extract of B. racemosa which exhibited promising anti Dalton's lymphoma ascitic in mice.[26] Previously, ethanol extract of B. racemosa leaves have also displayed cytotoxicity against the human cervical carcinoma cell (IC50 10 g/mL).[27]
CONCLUSION
Methanol fruit extracts of B. racemosa and H. sabdariffa exhibited varying degrees of total phenolic and flavonoid contents and antioxidant activity. The present study indicated that both phenolic and flavonoid compounds, known capable of oxidizing activities, play an important role in the antioxidant capability of these two plant species. Following this finding, further study is underway to identify important phytochemical bioactives for further development of this plant in nutraceutical and pharmaceutical use.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Norliyana Amran
Norliyana Amran, is a postgraduate student at the Universiti Sains Malaysia, where she graduated in Bachelor of Science (Plant Biotechnology) from Universiti Kebangsaan Malaysia in 2011. After gaining experience in Malaysian Agricultural Research and Development Institute for 3 months and Institute for Environment and Development for 6 months, she then joined an industrial training led by Agilent Technologies at IPHARM in 2012 for two years. Her research focused on the evaluation of anticancer of natural products, medicinal plants and analytical chemistry.

Anis Najwa Abdul Rani
Anis Najwa Abdul Rani, is a postgraduate student at the Pharmaceutical Chemistry Department of Universiti Sains Malaysia. She holds Bachelor of Science (Zoology) from Universiti Kebangsaan Malaysia in 2011 and has gaining her experience from the Veterinary Research Institute, Penang, Malaysia. She also worked as a pet care consultant in Pet Lovers Centre Pte Ltd (Malaysia) for a short period before joining a 2-years training program under the collaboration of Ministry of Science, Technology and Innovation of Malaysia and Agilent Technologies, which named the Bio-analytical Industrial Development Program in 2012. Her main focused on the research are the antidiabetic and cytotoxic research, natural product and analytical chemistry.

Roziahaniim Mahmud
Roziahanim Mahmud, obtained her B.Sc. in Chemistry from Sonoma State University, California USA in 1986. In 1990, she pursued her M.Sc. in Chemistry, in California State University, Long Beach, California USA. After gaining her master, she pursued her Ph.D. in Biochemical Toxicology at University of Liverpool, UK and completed her Ph.D. in 1996. She then worked as lecturer in Universiti Sains Malaysia until now. Her main focuses on the research are phytochemical and pharmacological studies of local medicinal plant.

Khoo Boon Yin
Khoo Boon Yin, obtained her B.Sc. in Applied Biology (Biotechnology) from Universiti Sains Malaysia in 1999. After gaining experience in the industry as a Microbiologist, she pursued her M.Sc. in Molecular Biology, in the same University, and obtained her M.Sc. in 2002. Little did she know that she was on a journey to Cancer Research, she pursued her Ph.D. in Medical Biotechnology at the Health Campus of the University in 2003 and completed her Ph.D. in 2007. She then worked as a temporary lecturer in INFORMM. Khoo pursued her post-doctoral training at the Division of Haematology & Oncology, Charité Campus Mitte, Humboldt University of Berlin in 2009. This training was funded by the Berliner Krebsgesellschaft e.V. in Germany. She returned to USM in 2010 and continued her journey as an academician in INFORMM until now
Acknowledgments
This research was financially supported by Universiti Sains Malaysia Research University (RU) Grant (1001/PFARMASI/812157) and Ministry of Science, Technology and Innovation of Malaysia under the Agilent Bio-analytical Industry Development Program grant. The authors would also like to acknowledge School of Pharmaceutical Sciences, Universiti Sains Malaysia.
REFERENCES
- 1.Takashima M, Horie M, Shichiri M, Hagihara Y, Yoshida Y, Niki E. Assessment of antioxidant capacity for scavenging free radicals in vitro: A rational basis and practical application. Free Radic Biol Med. 2012;52:1242–52. doi: 10.1016/j.freeradbiomed.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Baranisrinivasan P, Elumalai EK, Sivakumar C, Viviyan TS, David E. Hepatoprotective effect of Enicostemma littorale blume and Eclipta alba during ethanol induced oxidative stress in albino rats. Int J Pharmacol. 2009;5:268–72. [Google Scholar]
- 3.Akim AM, Ling LC, Rahmat A, Zakaria ZA. Antioxidant and anti-proliferative activities of Roselle juice on Caov-3, MCF-7, MDA-MB-231 and HeLa cancer cell lines. Afr J Pharm Pharmacol. 2011;5:957–65. [Google Scholar]
- 4.Omar AA, Ali ZM, Tamin NS. Malaysia: National Cancer Registry, Ministry of Health Malaysia; 2006. Malaysian Cancer Statistics - Data and Figure Peninsular Malaysia 2006; p. 8. [Google Scholar]
- 5.Naveen KD, Shikha S, Cijo-George V, Suresh PK, Ashok KR. Anticancer and anti-metastatic activities of Rheum emodi rhizome chloroform extracts. Asian J Pharm Clin Res. 2012;5:189–94. [Google Scholar]
- 6.Sim KS, Ibrahim H, Malek SN, Syamsir DR, Awang K. Cytotoxic activity of Alpinia murdochii Ridl: A mountain Ginger species from Peninsular Malaysia. Pharmacogn Mag. 2014;10:70–2. doi: 10.4103/0973-1296.126666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong HC. Kuala Lumpur, Malaysia: Utusan Publications and Distributors; 2004. Tumbuhan Liar: Khasiat Ubatan and Kegunaan Lain; pp. 160–3. [Google Scholar]
- 8.Nurul Mariam H, Radzali M, Johari R, Syahida A, Maziah M. Antioxidant activities of different aerial parts of Putat (Barringtonia racemosa L.) Malaysian J Biochem Mol Biol. 2008;16:15–9. [Google Scholar]
- 9.Hui-Hsuan L, Jing-Hsien C, Wu-Hsien K, Chau-Jong W. Chemo-preventive properties of Hibiscus sabdariffa L. on human gastric carcinoma cells through apoptosis induction and JNK/p38 MAPK signaling activation. Chem Biol Interact. 2007;165:59–75. doi: 10.1016/j.cbi.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–78. [Google Scholar]
- 11.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in Mulberry and their scavenging effects on superoxide radicals. J Food Chem. 1999;64:555–9. [Google Scholar]
- 12.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–8. [Google Scholar]
- 13.Tepe BD, Daferera D, Sokmen A, Sokmen M, Polissiou M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Laiaceae) J Food Chem. 2005;90:333–40. [Google Scholar]
- 14.Perumal S, Mahmud R, Piaru SP, Lee WC, Ramanathan S. Potential antiradical activity and cytotoxicity assessment of Ziziphus mauritiana and Syzygium polyanthum. Int J Pharmacol. 2012;8:535–41. [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Mohd-Esa N, Fong SH, Amin I, Chew LY. Antioxidant activity in different parts of Roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 2010;122:1055–60. [Google Scholar]
- 17.Obouayeba AP, Djyh NB, Diabate S, Djaman AJ, N’guessan JD, Kone M, et al. Phytochemical and antioxidant activity of Roselle (Hibiscus Sabdariffa L.) petal extracts. Res J Pharm Biol Chem Sci. 2014;5:1453. [Google Scholar]
- 18.Tupe RS, Kemse NG, Khaire AA. Evaluation of antioxidant potentials and total phenolic contents of selected Indian herbs powder extracts. Int Food Res J. 2013;20:1053–63. [Google Scholar]
- 19.Samanta SK, Bhattacharya K, Mandal C, Pal BC. Identification and quantification of the active component quercetin 3-O-rutinoside from Barringtonia racemosa, targets mitochondrial apoptotic pathway in acute lymphoblastic leukemia. J Asian Nat Prod Res. 2010;12:639–48. doi: 10.1080/10286020.2010.489040. [DOI] [PubMed] [Google Scholar]
- 20.Luo XD, Basile MJ, Kennelly EJ. Polyphenolic antioxidants from the fruits of Chrysophyllum crinito L. (star apple) J Agric Food Chem. 2002;50:1379–82. doi: 10.1021/jf011178n. [DOI] [PubMed] [Google Scholar]
- 21.Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–60. [Google Scholar]
- 22.Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–7. [Google Scholar]
- 23.Lopes F, Rocha A, Pirraco A, Regasini L, Silva D, Bolzani V, et al. Anti-angiogenic effects of pterogynidine alkaloid isolated from Alchornea glandulosa. BMC Complement Altern Med. 2009;9:1–11. doi: 10.1186/1472-6882-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010;116:164–76. doi: 10.1002/cncr.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talib WH, Mahasneh AM. Anti-proliferative activity of plant extracts used against cancer in traditional medicine. Sci Pharm. 2011;78:33–45. doi: 10.3797/scipharm.0912-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas TJ, Panikkar B, Subramoniam A, Nair MK, Panikkar KR. Antitumour property and toxicity of Barringtonia racemosa Roxb seed extract in mice. J Ethnopharmacol. 2002;82:223–7. doi: 10.1016/s0378-8741(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 27.Mackeen MM, Ali AM, El-Sharkawy SH, Manap MY, Salleh KM, Lajis NH, et al. Antimicrobial and cytotoxic properties of some Malaysian traditional vegetables. Int J Pharmacognosy. 1997;35:174–8. [Google Scholar]


