Abstract
Objective
Innovative screening methods such as self-testing for human papillomavirus (HPV) may alleviate barriers to cervical cancer screening. The purpose of this exploratory study was to determine whether Appalachian Kentucky women would be amenable to self-collecting a cervico-vaginal specimen for HPV testing.
Methods
Women aged 30–64 who were overdue for guideline-recommended cervical cancer screening were recruited from a primary care clinic in southeastern Kentucky. The women were asked to self-collect a specimen, using a cervico-vaginal brush, based on verbal and printed directions provided by a research nurse. All study participants, regardless of laboratory-confirmed HPV status, received the same counseling on the importance of cervical cancer screening and offered navigation to follow-up Pap testing at the local health department.
Results
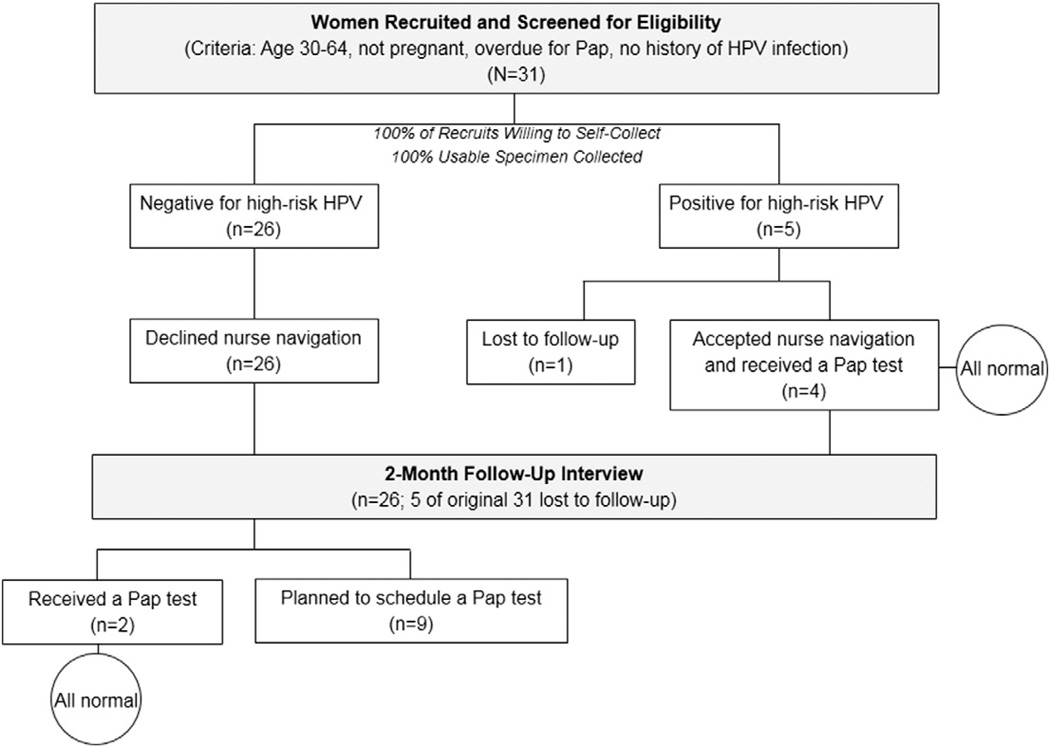
Thirty-one women were approached and recruited to participate in the study, indicating a 100% acceptance rate of HPV self-testing. Of the 31 women, 26 tested negative for high-risk HPV and five tested positive. All of the women with negative results declined nurse navigation to Pap testing, whereas four of the five women with positive results accepted nurse navigation and received subsequent Pap smear screenings (all results were normal).
Conclusions
Among this sample of Appalachian Kentucky women, self-collecting a cervico-vaginal specimen for HPV testing was highly acceptable. This exploratory study provides impetus for larger studies among high-risk, medically underserved women in rural communities. Tailoring alternative cancer screening strategies to meet the complex needs of rural women is likely to lead to reductions in cervical cancer incidence and mortality among this vulnerable population.
Keywords: Cervical cancer, HPV testing, Appalachia, Acceptance, Patient navigation
Introduction
Despite being one of the few cancers that is theoretically 100% preventable, about 12,000 new cases of invasive cervical cancer (ICC) are diagnosed annually in the U.S. [1]. According to the National Cancer Institute, in 2009, the incidence rate of ICC was 7.9 per 100,000 women [2], an endemic level that remains an intractable challenge to public health, particularly in rural communities. Women who reside in rural areas have consistently higher incidence and mortality rates of this disease compared to their urban counterparts [3]. More specifically, residents of Appalachia have been disproportionately affected by this malignancy [4–7]. Even within Appalachia, certain geographical areas are more heavily burdened with ICC. For example, higher mortality rates have been found in the Appalachian region of Kentucky compared to Appalachian regions of other states [5,7,8]. The Kentucky Cancer Registry (2006–2010) reports an increased age-adjusted ICC mortality rate in Appalachian counties (9.76 per 100,000) compared to non-Appalachian counties (8.47 per 100,000) within the state [9].
Contributing to the increased burden of ICC in rural Appalachia is lower Papanicolaou (Pap) testing rates [10–12]. In 2010, the majority of Appalachian Kentucky counties reported lower rates of women aged 18 and over receiving a Pap test within the past 3 years (74.3%–78.3%) compared to state (80.9%) and national (81.1%) estimates [13]. Lower rates of Pap testing among this population are concerning because routine screening for cervical abnormalities, combined with effective treatment, has been shown to dramatically decrease the risk of developing ICC. For example, approximately 60% of all new ICC cases in the U.S. occur among women who are rarely or never screened [14]. Low Pap testing rates among rural, Appalachian women are associated with various factors, including older age, lack of health insurance, transportation barriers, lack of childcare, tobacco use, and lower levels of income and education [10–12,15–17]. Furthermore, community-level healthcare provider shortages, lack of a medical home, travel distances and clinic hours, embarrassment, discomfort with male providers, and lack of continuity of care influence Appalachian women's decisions to obtain Pap tests [10,11,15–19].
Due to the numerous barriers that prevent Appalachian women from obtaining routine Pap testing, the need for a convenient cervical cancer screening method is apparent. The high-risk (oncogenic) human papillomavirus (HR-HPV) test [20,21] is a highly efficient method of screening that women may be able to perform themselves in the privacy of their home or other private location, thereby averting clinic-based barriers to initial screening [22]. A recent review of the literature revealed that self-collected cervico-vaginal samples for high-risk HPV testing are comparable to physician-collected samples in terms of accuracy in detecting cervical intraepithelial neoplasia grade 2 (CIN2) or higher [23].
Given the advent of self-collection methods as an initial screening option for ICC, an important research question involves acceptability of self-collection among various populations of at-risk women. To date, studies have largely established high rates of reported acceptance for self-administered cervico-vaginal specimen collection among various populations of women [24,25]. A review conducted in 2011 found that studies reported acceptance rates of 75–93% [25]. Furthermore, studies have shown high acceptability among women living in low-resource settings both domestically and abroad [26–30]. However, the majority of studies have been conducted in urban areas; the question of acceptability for self-collected methods for cervical cancer screening has yet to be addressed among medically underserved women living in rural Appalachia.
Accordingly, the purpose of this exploratory study was to determine whether Appalachian Kentucky women would be amenable to self-collecting a cervico-vaginal specimen for HPV testing. A secondary purpose of the study was to implement and track the results of a patient counseling and navigation intervention promoting guideline-recommended cervical cancer Pap smear screening among all women participating in the study.
Methods
Study sample
Recruitment took place at a free primary care clinic in southeastern Kentucky. The clinic provides care to residents of some of the poorest and most medically underserved counties in the U.S. [31,32]. Many of the patients are without health insurance. An advanced practice registered nurse (APRN) employed by the University of Kentucky Rural Cancer Prevention Center recruited potential study participants at the clinic on six separate days in November 2011. The nurse answered questions about the study, screened women for study participation, obtained informed consent and baseline data, and provided both verbal and visual self-collection directions. Women were eligible to be in the study if they were between the ages of 30 and 64, were not pregnant, reported not having a Pap test in the past four years, and had never tested positive for HPV infection. Participants were also asked to complete a HIPAA release form, which provided researchers access to specific medical information (i.e., follow-up Pap smear results) needed for study evaluation.
Data collection
After provision of informed consent, women completed a brief, self-administered questionnaire written at a sixth-grade reading level. Items assessed socio-demographics, smoking status, and sexual behaviors, including number of lifetime male sex partners, number of male sex partners within the past 12 months, and knowledge of whether their male partners had concurrent sex partners. Women also provided information about their past experiences with Pap tests, HPV knowledge, and perceived barriers to gynecological care. Each participant received a $25 gift card at the time of interview as compensation for their time. This study was approved by the University of Kentucky Institutional Review Board.
Testing for HPV
The Hybrid Capture® 2 High-Risk HPV DNA Test® (Qiagen Corporation; Gaithersburg, MD)was used for the study. This test provides confirmation of the presence of one or more of 13 high-risk types of HPV infection (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). Women were verbally instructed by the nurse on the correct method for self-collecting a cervico-vaginal specimen. In addition, participants were given a graphic-based instruction sheet for use in the clinic restroom during the self-collection process. Women were instructed to insert a Fisherbrand* Cervex-Brush* Cervical Cell Sampler (Thermo Fisher Scientific Inc.; Waltham, MA) into the cervico-vaginal canal much like a tampon, until meeting resistance, rotate the brush five times, remove it slowly, and place it in the screw-top vial containing Scope® mouth-wash. Specimens were labeled and sent to a laboratory (Cleveland Clinic Laboratories; Cleveland, OH) for analysis. All women were notified of their test results in person or by telephone by the nurse within 24 h of receiving the results from the laboratory.
Patient counseling and navigation
At the time of results notification, regardless of their HPV test results status, women were counseled on the importance of receiving routine, guideline-appropriate cervical cancer screening according to the U.S. Preventive Services Task Force's recommendations [33]. Additionally, all women were offered navigation services from the APRN, including help scheduling a Pap test at a local health department and assistance with transportation, childcare, and personal support if needed.
Lastly, the APRN conducted follow-up interviews two months post-results notification with all study participants to answer any additional questions about their results, inquire about whether or not they discussed their HPV test results with friends, family members, and/or sex partner(s), and ascertain whether or not a subsequent Pap test appointment had been made (and results if applicable).
Results
During recruitment for this pilot study, a total of 31 women were approached and recruited to participate. None of the women approached for participation declined, and all successfully completed the self-collected cervico-vaginal brush, indicating a 100% acceptance rate of this new high-risk HPV screening method among this sample of Appalachian Kentucky women (Fig. 1). As presented in Table 1, the mean age of the sample was 38.5 years (SD = 7.6). All participants self-identified as Caucasian. More than three-quarters (77.4%) of the women reported an annual household income of <$25,000 and 40% did not have health insurance. Just over half of the women (58.1%) were married at the time of the baseline interview. Most women reported current cigarette smoking (73.3%). The mean number of lifetime male sex partners was 7.06 (SD = 5.6); five participants (16.7%) indicated that a male partner may have had concurrent sex with another partner during their time together. Three women (9.7%) reported never having a Pap test in their lifetime. Among those reporting at least one Pap test, six (19.4%) indicated having at least one abnormal result. Almost 90% of the women knew that cervical cancer is caused by HPV. Thirty percent of the sample indicated that they had minimal trust in doctors and the healthcare system, and that they did not have time to see a gynecologist. Forty percent of the women were “very afraid” of developing cervical cancer.
Fig. 1.
Study design and results.
Table 1.
Participant characteristics (N = 31).
| Characteristic | N (%) |
|---|---|
| Age (years) | |
| Mean (SD) | 38.5 (7.6) |
| Range | 29–58 |
| Race/ethnicity | |
| Caucasian | 31 (100) |
| Income | |
| <$25,000 | 24 (77.4) |
| >$25,000 | 7 (22.6) |
| Health insurance (n = 30) | |
| Yes | 17 (56.7) |
| No | 13 (43.3) |
| Marital status | |
| Single | 8 (25.8) |
| Married | 18 (58.1) |
| Separated/divorced/widowed | 5 (16.1) |
| Current cigarette use (n = 30) | |
| Yes | 22 (73.3) |
| No | 8 (26.7) |
| Lifetime male sex partners (number) | |
| Mean (SD) | 7.06 (5.6) |
| Range | 0–20 |
| Past 12 months male sex partners (number) | |
| Mean (SD) | 1.13 (.56) |
| Range | 0–3 |
| Sex with male who had concurrent partners (n = 30) | |
| Yes | 5 (16.7) |
| No | 26 (83.3) |
| Ever had a Pap smear | |
| Yes | 28 (90.3) |
| No | 3 (9.7) |
| Cervical cancer knowledge and perceived barriers to gynecological care | |
| Cervical cancer is caused by HPV | 27 (87.1) |
| Some or very little trust in doctors and healthcare system (n = 30) | 9 (30.0) |
| No time to visit a gynecologist | 9 (29.0) |
| Very afraid of developing cervical cancer | 12 (38.7) |
Of the 31 women providing self-collected specimens, five (16.1%) tested positive for one of thirteen types of high-risk HPV types. None of the specimens yielded indeterminate findings, thereby suggesting that women used the proper technique for self-collection. None of the measures assessed in the self-administered questionnaire approached significance in testing possible associations with a high-risk HPV-positive result. Being single (P = .58), suspecting that a sex partner had concurrent partners (P = 1.00), ever having an abnormal Pap (P = .24), and current use of tobacco (P = .61) each yielded weak differences between groups. All five women testing positive for high-risk HPV reported a history of Pap testing; two of these women specifically reported a history of an abnormal Pap.
In assessing the impact of the patient counseling and navigation process which was identical for all study participants, at results notification, all 26 women testing negative for high-risk HPV declined nurse-assisted navigation to follow-up Pap testing. In contrast, four of the five women testing positive for high-risk HPV accepted navigation to the local health department for follow-up Pap testing. The fifth positive woman was lost to follow-up due to incarceration. All four women's follow-up Pap tests were normal as confirmed through medical record review.
Twenty-six of the 31 women (83.9%) were interviewed two months post-results notification; five women were lost to follow-up. Overall, women were more likely to discuss their HPV results with a family member (n = 15) than a sex partner (n = 6) or a female friend (n = 7). Even though all of the women testing negative for high-risk HPV initially declined navigation to Pap testing, at the two-month follow-up, two of these women had received a Pap test (with normal results) and nine indicated they were planning to make a future appointment.
Discussion
This novel, exploratory study found 100% acceptance of self-collecting cervico-vaginal specimens for HPV testing among a high-risk population of medically underserved women in Appalachian Kentucky. This acceptance rate is higher than previous studies examining acceptability of self-collected methods in rural regions of the U.S. [30,34,35]. We believe we had a higher acceptance rate for several reasons. First, we perceive women found self-collection to be a favorable alternative to provider-performed Pap test due to its convenience, ease, privacy, brevity, and less invasive nature. Second, these women were attending a trusted healthcare clinic which provides many services for free or low-cost. In turn, women may have been more amenable to receiving a free health screening through our research study. Third, at the time of this study, the clinic did not offer Pap testing (patients were referred to the local health department); simultaneously, the women were also aware they were overdue for traditional Pap testing. Therefore, our “real time” offer of cervical cancer screening in the clinic – albeit with an alternative method – may have served as the tipping point for participation. Fourth, the APRN is indigenous to the community, living there all of her life. She is a trusted healthcare provider in the area and relates well to community members. Last, several of the women who participated in the study during the first few days of recruitment encouraged family members (e.g., mother-daughter, sister-sister), coworkers, and other acquaintances to participate due to their own favorable experience with the self-collection procedure and knowledge that their peers were also overdue for this important cancer screening test.
In the current study, approximately one of every six women (16.7%) tested positive for at least one high-risk type of HPV. Nationally, the prevalence of high-risk HPV infection for women ages 14–59 is 29% [36]. Importantly, a majority of the participants reported current cigarette use (73.3%). Tobacco use is known to be associated with a higher risk of cervical cancer among HPV-positive women [37] and noncompliance with Pap testing [12,38,39]; thus, women who use tobacco heighten their risk for ICC.
The navigation component of this study found that women are generally willing to receive a Pap test (or make a future appointment) after being informed of their HPV status and receiving counseling on the importance of guideline-appropriate cervical cancer screening. Interestingly, acceptance of nurse navigation was higher among those testing positive for high-risk HPV than those who tested negative in our relatively small sample. Women with positive results may perceive themselves at higher risk for ICC and thus be more likely to engage in protective behaviors such as Pap testing. These results are promising in that a recent study found that women who utilize a patient navigator typically have higher resolution rates (i.e., determination of a benign or malignant condition) within the first six months of an abnormal cervical screening, including ICC [40].
Clearly, this pilot study provides the impetus for larger scale studies of cervico-vaginal self-brush collection for HPV testing among high-risk, medically underserved women in rural communities. Given the cultural, socioeconomic, and healthcare barriers associated with lower Pap testing rates among rural women, these initial findings suggest that self-collected brush screenings may be useful for this population as an initial screening, as has been previously established in other low-resource areas. If HPV self-collection were to gain approval for screening in the U.S. in the future, this would allow for focused triage of high-risk HPV-positive women into follow-up Pap smear screening. Following screening guidelines, HPV-negative women would be able to extend their screening intervals to 3–5 years depending on age and previous cervical cancer screening results [33]. Although none of the high-risk HPV-positive women in our study who had subsequent Pap smears were found to have abnormal cytology, it is likely that a greater number of cases would be identified in the application of this two-stage screening method (self-collection followed by Pap smear testing) in a larger sample or in clinical practice over time. Even a small number of high-grade cervical intraepithelial neoplasia (CIN) case findings would be likely to provide substantial cost-savings given the advantages of early detection and associated treatment. Indeed, potential discovery of cervical abnormalities following a positive high-risk HPV test via self-collection could help lessen the $1.2 billion direct [and $1.8 billion indirect] medical costs associated with treating cervical abnormalities and malignancies each year in the U.S. [41,42].
Noted limitations to this pilot study include the small sample size and use of a convenience sample. The study sample was also limited in racial and ethnic diversity, reflecting the composition of the Appalachian Kentucky population. Therefore, the results may not be generalizable to all rural or Appalachian populations. Results of the Hybrid Capture® 2 High-Risk HPV DNA Test® only provide confirmation of one or more of 13 high-risk types of HPV. Assay results do not confirm the presence of low-risk HPV types such as 6 or 11 known to cause genital warts, nor do they confirm negative HPV infection. Additionally, the data collected via baseline and follow-up interviews were primarily based on self-report. Previous studies have noted that women tend to overstate their participation in cervical cancer screenings [43]. There is also the possibility for selection bias as study participants were already seeking services at a healthcare clinic and may be more proactive in accepting cervical cancer screening. However, despite a clinic-based setting, all 31 women verbally confirmed they were overdue for routine cervical cancer screening by at least four years, though we did not assess the exact number of years they were overdue. The study design also did not allow for a comparison of acceptance rates with women who were guideline-compliant with cervical cancer screening. Lastly, we did not directly assess the women's experience with self-collection via survey or indepth qualitative inquiry. However, the APRN received anecdotal comments indicating a favorable experience with the self-collection process and preference over a traditional, provider-performed Pap test. Several women inquired about the possibility of performing the self-collection strategy annually in their own homes.
Despite these limitations, our study tested a two-stage process – self-collection for HPV testing, followed by patient navigation to clinical services – which focused on eliminating barriers to cervical cancer screening among an at-risk population of women in a medically underserved, rural region of Kentucky. Women in this area are disproportionately burdened by cervical cancer incidence and mortality compared to women in non-Appalachian Kentucky and other regions of the U.S. The well-documented barriers to cervical cancer screening among rural women necessitate creative and efficacious solutions. Consequently, tailoring alternative cancer screening strategies to meet the complex needs of medically underserved rural women is likely to lead to reductions in incidence, morbidity, and mortality rates of cervical cancer and related medical costs among this vulnerable population.
Acknowledgments
The authors would like to thank Tom Collins, BS, Wallace Bates, MBA, Scott Shapiro, MD, and the staff of the Little Flowers Clinic for their assistance with the pilot study, including study design, data collection, and participant recruitment.
This publication was supported by Cooperative Agreement Number 1U48DP001932-01 from the Centers for Disease Control and Prevention. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Dr. Jennifer Smith has received research grants, served on paid advisory boards, and/or been a paid speaker for Hologic Gen-Probe and QIAGEN. Dr. Richard Crosby served as a paid consultant for Merck Pharmaceuticals in 2012. Drs. Richard Crosby and Robin Vanderpool are co-investigators on an investigator-initiated research project awarded to the University of Kentucky (E. Cohen, PI; MISP# 50154, 2013–2015) by Merck Pharmaceuticals.
Footnotes
Conflict of interest statement
Three co-authors reported declarations of conflicting interests.
References
- 1.Centers for Disease Control, Prevention (US) Cervical cancer fact sheet. [Internet] Atlanta (GA): U S Department of Health and Human Services; 2012. [cited 2013 May 9]. Available from: http://www.cdc.gov/cancer/cervical/pdf/cervical_facts.pdf. [Google Scholar]
- 2.National Cancer Institute (US) State cancer profiles [Internet] Bethesda (MD): US Department of Health and Human Services, National Institutes of Health; 2013. May 09, [cited 2013 May 9]. Available from: http://statecancerprofiles.cancer.gov/cgi-bin/quickprofiles/profile.pl?00&057#incdEAPC. [Google Scholar]
- 3.Yabroff KR, Lawrence WF, King JC, Mangan P, Washington KS, Yi B, et al. Geographic disparities in cervical cancer mortality: what are the roles of risk factor prevalence, screening, and use of recommended treatment? J Rural Health Spring. 2005;21(2):149–157. doi: 10.1111/j.1748-0361.2005.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 4.Wingo PA, Tucker TC, Jamison PM, Martin H, McLaughlin C, Bayakly R, et al. Cancer in Appalachia, 2001–2003. Cancer. 2008 Jan 1;112(1):181–192. doi: 10.1002/cncr.23132. [DOI] [PubMed] [Google Scholar]
- 5.Hopenhayn C, King JB, Christian A, Bin H, Christian WJ. Variability of cervical cancer rates across 5 Appalachian states, 1998–2003. Cancer. 2008 Nov 15;113(10 Suppl.):2974–2980. doi: 10.1002/cncr.23749. [DOI] [PubMed] [Google Scholar]
- 6.Lengerich EJ, Tucker TC, Powell RK, Colsher P, Lehman E, Ward AJ, et al. Cancer incidence in Kentucky, Pennsylvania, and West Virginia: disparities in Appalachia. J Rural Health Winter. 2005;21(1):39–47. doi: 10.1111/j.1748-0361.2005.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control, Prevention (US) Cancer death rates—Appalachia, 1994–1998. MMWR Morb Mortal Wkly Rep. 2002 Jun 21;51(24):527–529. [PubMed] [Google Scholar]
- 8.Paskett ED, Fisher JL, Lengerich EJ, Schoenberg NE, Kennedy SK, Conn ME, et al. Disparities in underserved white populations: the case of cancer-related disparities in Appalachia. Oncologist. 2011;16(8):1072–1081. doi: 10.1634/theoncologist.2011-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kentucky Cancer Registry (US) Invasive cancer incidence rates by Appalachian region in Kentucky cervix uteri; 2006–2010 [Internet. Lexington (KY): [cited 2013 May 9]. Available from: http://cancer-rates.info/ky/index.php. [Google Scholar]
- 10.Hall HI, Uhler RJ, Coughlin SS, Miller DS. Breast and cervical cancer screening among Appalachian women. Cancer Epidemiol Biomarkers Prev. 2002 Jan;11(1):137–142. [PubMed] [Google Scholar]
- 11.Hatcher J, Studts CR, Dignan MB, Turner LM, Schoenberg NE. Predictors of cervical cancer screening for rarely or never screened rural Appalachian women. J Health Care Poor Underserved. 2011 Feb;22(1):176–193. doi: 10.1353/hpu.2011.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amonkar MM, Madhavan S. Compliance rates and predictors of cancer screening recommendations among Appalachian women. J Health Care Poor Underserved. 2002 Nov;13(4):443–460. doi: 10.1353/hpu.2010.0582. [DOI] [PubMed] [Google Scholar]
- 13.Kentucky Cabinet for Health, Human Services (US) Frankfort (KY): 2011. [cited 2013 May 9]. Kentucky Area Development District (ADD) profiles: 2010 Behavioral Risk Factor Surveillance System (BRFSS) [Internet, Available from: http://chfs.ky.gov/NR/rdonlyres/55B0D404-F321-4D2D-B913-991B41BFDBB4/0/2010KYADDProfiles.pdf] [Google Scholar]
- 14.American Cancer Society (US) Cervical cancer. Atlanta (GA): 2013. [cited 2013 May 11]. [Internet, Available from: http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-pdf. [Google Scholar]
- 15.Schoenberg NE, Hopenhayn C, Christian A, Knight EA, Rubio A. An in-depth and updated perspective on determinants of cervical cancer screening among central Appalachian women. Women Health. 2005;42(2):89–105. doi: 10.1300/j013v42n02_06. [DOI] [PubMed] [Google Scholar]
- 16.Katz ML, Wewers ME, Single N, Paskett ED. Key informants' perspectives prior to beginning a cervical cancer study in Ohio Appalachia. Qual Health Res. 2007 Jan;17(1):131–141. doi: 10.1177/1049732306296507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyttle NL, Stadelman K. Assessing awareness and knowledge of breast and cervical cancer among Appalachian women. Prev Chronic Dis. 2006 Oct;3(4):A125. [PMC free article] [PubMed] [Google Scholar]
- 18.Coughlin SS, Thompson TD, Hall HI, Logan P, Uhler RJ. Breast and cervical carcinoma screening practices among women in rural and nonrural areas of the United States, 1998–1999. Cancer. 2002 Jun 1;94(11):2801–2812. doi: 10.1002/cncr.10577. [DOI] [PubMed] [Google Scholar]
- 19.Studts CR, Tarasenko YN, Schoenberg NE, Shelton BJ, Hatcher-Keller J, Dignan MB. A community-based randomized trial of a faith-placed intervention to reduce cervical cancer burden in Appalachia. Prev Med. 2012 Jun;54(6):408–414. doi: 10.1016/j.ypmed.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castle PE, Stoler MH, Wright TC, Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011 Sep;12(9):880–890. doi: 10.1016/S1470-2045(11)70188-7. [DOI] [PubMed] [Google Scholar]
- 21.Cox JT, Castle PE, Behrens CM, Sharma A, Wright TC, Jr, Cuzick J. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: results from the ATHENA HPV study. Am J Obstet Gynecol. 2013 Mar;208(3):e1–e11. doi: 10.1016/j.ajog.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Gok M, Heideman DA, van Kemenade FJ, Berkhof J, Rozendaal L, Spruyt JW, et al. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ. 2010;340:c1040. doi: 10.1136/bmj.c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snijders PJ, Verhoef VM, Arbyn M, Ogilvie G, Minozzi S, Banzi R, et al. High-risk HPV testing on self-sampled versus clinician-collected specimens: a review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer. 2013 May 15;132(10):2223–2236. doi: 10.1002/ijc.27790. [DOI] [PubMed] [Google Scholar]
- 24.Huynh J, Howard M, Lytwyn A. Self-collection for vaginal human papillomavirus testing: systematic review of studies asking women their perceptions. J Low Genit Tract Dis. 2010 Oct;14(4):356–362. doi: 10.1097/LGT.0b013e3181dc115b. [DOI] [PubMed] [Google Scholar]
- 25.Schmeink CE, Bekkers RL, Massuger LF, Melchers WJ. The potential role of self-sampling for high-risk human papillomavirus detection in cervical cancer screening. Rev Med Virol. 2011 May;21(3):139–153. doi: 10.1002/rmv.686. [DOI] [PubMed] [Google Scholar]
- 26.Barbee L, Kobetz E, Menard J, Cook N, Blanco J, Barton B, et al. Assessing the acceptability of self-sampling for HPV among Haitian immigrant women: CBPR in action. Cancer Causes Control. 2010 Mar;21(3):421–431. doi: 10.1007/s10552-009-9474-0. [DOI] [PubMed] [Google Scholar]
- 27.Dzuba IG, Diaz EY, Allen B, Leonard YF, Lazcano Ponce EC, Shah KV, et al. The acceptability of self-collected samples for HPV testing vs. the Pap test as alternatives in cervical cancer screening. J Womens Health Gend Based Med. 2002 Apr;11(3):265–275. doi: 10.1089/152460902753668466. [DOI] [PubMed] [Google Scholar]
- 28.Guan Y, Castle PE, Wang S, Li B, Feng C, Ci P, et al. A cross-sectional study on the acceptability of self-collection for HPV testing among women in rural China. Sex Transm Infect. 2012 Nov;88(7):490–494. doi: 10.1136/sextrans-2012-050477. [DOI] [PubMed] [Google Scholar]
- 29.Quincy BL, Turbow DJ, Dabinett LN. Acceptability of self-collected human papillomavirus specimens as a primary screen for cervical cancer. J Obstet Gynaecol. 2012 Jan;32(1):87–91. doi: 10.3109/01443615.2011.625456. [DOI] [PubMed] [Google Scholar]
- 30.Scarinci IC, Litton AG, Garces-Palacio IC, Partridge EE, Castle PE. Acceptability and usability of self-collected sampling for HPV testing among African-American women living in the Mississippi Delta. Womens Health Issues. 2013 Mar-Apr;23(2):e123–e130. doi: 10.1016/j.whi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Health Resources and Services Administration (US) Medically Underserved Areas and Populations. [Internet] Rockville (MD): U S Department of Health and Human Services; 2013. [cited 2013 July 26]. Available from: http://www.hrsa.gov/shortage/find.html. [Google Scholar]
- 32.Appalachian Regional Commission (US) County Economic Status, Fiscal Year 2014. Washington D.C.: 2013. [cited 2013 July 26]. [Internet, Available from: http://www.arc.gov/reports/custom_report.asp?REPORT_ID=45] [Google Scholar]
- 33.U.S. Preventive Servcies Task Force (US) Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement [Internet] Rockville (MD): 2012. Mar, [cited 2013 May 11]. Available from: http://www.uspreventiveservicestaskforce.org/uspstf11/cervcancer/cervcancerrs.htm. [Google Scholar]
- 34.Castle PE, Rausa A, Walls T, Gravitt PE, Partridge EE, Olivo V, et al. Comparative community outreach to increase cervical cancer screening in the Mississippi Delta. Prev Med. 2011 Jun;52(6):452–455. doi: 10.1016/j.ypmed.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper DM, Noll WW, Belloni DR, Cole BF. Randomized clinical trial of PCR-determined human papillomavirus detection methods: self-sampling versus clinician-directed—biologic concordance and women's preferences. Am J Obstet Gynecol. 2002 Mar;186(3):365–373. doi: 10.1067/mob.2002.121076. [DOI] [PubMed] [Google Scholar]
- 36.Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011 Aug 15;204(4):566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 37.Plummer M, Herrero R, Franceschi S, Meijer CJ, Snijders P, Bosch FX, et al. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case–control study. Cancer Causes Control. 2003 Nov;14(9):805–814. doi: 10.1023/b:caco.0000003811.98261.3e. [DOI] [PubMed] [Google Scholar]
- 38.Wyatt SW, Lancaster M, Bottorff D, Ross F. History of tobacco use among Kentucky women diagnosed with invasive cervical cancer: 1997–1998. J Ky Med Assoc. 2001 Dec;99(12):537–539. [PubMed] [Google Scholar]
- 39.Centers for Disease Control, Prevention (US) Women and smoking: a report of the Surgeon General. [Internet] Atlanta (GA): U S Department of Health and Human Services; 2001. Mar, [cited 2013 July 29]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK44303/ [Google Scholar]
- 40.Paskett ED, Katz ML, Post DM, Pennell ML, Young GS, Seiber EE, et al. The Ohio Patient Navigation Research Program: does the American Cancer Society patient navigation model improve time to resolution in patients with abnormal screening tests? Cancer Epidemiol Biomarkers Prev. 2012 Oct;21(10):1620–1628. doi: 10.1158/1055-9965.EPI-12-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012 Sep;30(42):6016–6019. doi: 10.1016/j.vaccine.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Cancer Institute (US) Bethesda (MD): U S Department of Health and Human Services, National Institutes of Health; 2012. [cited 2013 July 26]. Cancer Trends Progress Report — 2011/2012 Update. [Internet] Available from: http://progressreport.cancer.gov/ [Google Scholar]
- 43.Howard M, Lytwyn A, Lohfeld L, Redwood-Campbell L, Fowler N, Karwalajtys T. Barriers to acceptance of self-sampling for human papillomavirus across ethnolinguistic groups of women. Can J Public Health. 2009 Sep-Oct;100(5):365–369. doi: 10.1007/BF03405272. [DOI] [PMC free article] [PubMed] [Google Scholar]