Abstract
Community-acquired pneumonia (CAP) is a common but potentially life-threatening condition, but limited information exists on the effectiveness of fluoroquinolones compared to β-lactams in outpatient settings. We aimed to compare the effectiveness and outcomes of penicillins versus respiratory fluoroquinolones for CAP at outpatient clinics.
This was a claim-based retrospective cohort study. Patients aged 20 years or older with at least 1 new pneumonia treatment episode were included, and the index penicillin or respiratory fluoroquinolone therapies for a pneumonia episode were at least 5 days in duration. The 2 groups were matched by propensity scores. Cox proportional hazard models were used to compare the rates of hospitalizations/emergence service visits and 30-day mortality. A logistic model was used to compare the likelihood of treatment failure between the 2 groups.
After propensity score matching, 2622 matched pairs were included in the final model. The likelihood of treatment failure of fluoroquinolone-based therapy was lower than that of penicillin-based therapy (adjusted odds ratio [AOR], 0.88; 95% confidence interval [95%CI], 0.77–0.99), but no differences were found in hospitalization/emergence service (ES) visits (adjusted hazard ratio [HR], 1.27; 95% CI, 0.92–1.74) and 30-day mortality (adjusted HR, 0.69; 95% CI, 0.30–1.62) between the 2 groups.
The likelihood of treatment failure of fluoroquinolone-based therapy was lower than that of penicillin-based therapy for CAP on an outpatient clinic basis. However, this effect may be marginal. Further investigation into the comparative effectiveness of these 2 treatment options is warranted.
INTRODUCTION
Community-acquired pneumonia (CAP) is a common but potentially life-threatening condition. The estimated mortality rate for CAP ranges from 5.1% for patients in ambulatory care settings to 36.5% for patients in intensive care units.1 The initial treatment for CAP is often empirical rather than target therapy because CAP can be caused by a wide variety of pathogens, and it takes time to identify the causative pathogens. Therefore, it is a challenge for clinicians to select an empirical regimen that has the spectrum and potency to cover the potential causative pathogens while minimizing the risk of drug resistance and treatment failure.
The current recommendations in Taiwan for outpatient CAP empirical treatments include penicillins, macrolides, respiratory fluoroquinolones, or a combination of penicillins and macrolides.2 Although β-lactams cover most of the common pathogens for CAP, a lack of coverage of atypical pathogens and potential microbial resistance to these antibiotics, such as penicillin-resistant Streptococcus pneumoniae, may be a concern.3–6 Respiratory fluoroquinolones, such as levofloxacin, moxifloxacin, and gemifloxacin cover a broader spectrum, have higher potency, and penetrate better into the respiratory tract.7,8 Surveillance data have also revealed lower rates of drug resistance among common respiratory pathogens associated with fluoroquinolones (<2%).4,9 In addition, the enhanced pharmacokinetic profile allows respiratory fluoroquinolones to be administered once daily, providing an optimal alternative for CAP treatment.7,8
Although many studies have compared the efficacy of fluoroquinolones to that of β-lactams (±macrolides) among inpatient settings,8,10–16 information from outpatient settings is limited. Using a U.S. claims database, one study showed that, in outpatient settings, the rates of treatment failure and emergence service (ES) visits of patients treated with fluoroquinolones were lower than those of patients treated with macrolides, but no differences were found for CAP-related hospitalizations or treatment costs.17 Although the U.S. guidelines recommend fluoroquinolones for patients with comorbidities, those at risk of penicillin-resistant Streptococcus pneumoniae, and those aged ≥65,18,19 fluoroquinolones are commonly prescribed for uncomplicated and elderly patients with pneumonia in ambulatory care.20 Therefore, better understanding of the effectiveness of fluoroquinolones for outpatient CAP treatment is needed. The purpose of this study is to compare the effectiveness of a fluoroquinolone-based regimen to a penicillin-based regimen using a nationwide population-based cohort in Taiwan. We hypothesized that fluoroquinolone-based therapy would be associated with better treatment outcomes as compared to penicillin-based therapy. The findings of this study will contribute evidence on the effectiveness of fluoroquinolones for outpatient pneumonia treatment, especially in an Asian population.
METHODS
Ethics Statement
This study was conducted in Taiwan only, and Institutional Review Board (IRB) approval was obtained from the Institutional Review Board of National Taiwan University Hospital (NTUH-REC No. 201501011W). This was a retrospective database review without intervention or obtaining extra clinical specimens. Human specimens were not directly used in this research and informed consent was waived. The waiving of informed consent was approved by the Institutional Review Board of National Taiwan University Hospital.
Data Source
Data for this study were gathered from the 2002 to 2011 National Health Insurance Research Database (NHIRD), which contains administrative claims data from approximately 23 million enrollees (more than 99.9% of the population) under the National Health Insurance (NHI) program in Taiwan. To protect privacy and improve computation efficiency, the data in this study were drawn from 3 subsets of the NHIRD: the 2000, 2005, and 2010 Longitudinal Health Insurance Databases (LHIDs). Each of the LHIDs is comprised of 1 million randomly selected samples from beneficiaries enrolled in the years of 2000, 2005, and 2010, respectively, and the LHID samples are considered to be nationally representative.21 All of the LHID files contain deidentified information on the beneficiaries’ enrollment, inpatient and outpatient service utilization, and prescription drugs.
Study Design and Sample
This claim-based retrospective cohort study assessed the effectiveness of penicillin-based and respiratory fluoroquinolone-based therapies for CAP in outpatient settings. Patients aged 20 years or older with at least 1 new pneumonia treatment episode were included. A pneumonia episode was defined as an outpatient visit with a primary diagnosis of pneumonia (The International Classification of Diseases, Ninth Revision, Clinical Modification: 481–483, 484.8, 485–486, 487.0) and a confirmatory diagnostic procedure, such as chest X-ray, sputum culture, or blood culture. Also required for inclusion was a prescription of at least 5 days of oral fluoroquinolone monotherapy (of levofloxacin, moxifloxacin, or gemifloxacin) or oral penicillin therapy issued on the date of the pneumonia diagnosis. For the penicillin group, macrolides were the only antibiotic treatment coprescribed on the index date. Penicillins were chosen for comparison because they are recommended as the first-line empirical treatment for outpatient CAP in Taiwan. A treatment episode was considered to be newly initiated if the patient had not used any antibiotics (including antituberculosis drugs) within 30 days before the index pneumonia diagnosis (ie, the index date). Treatment episodes were excluded if the patient had been hospitalized within 30 days before the index date, had human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) or a transplant before the index date, had used chemotherapy or immune target therapy (eg, antilymphocyte globulin, antithymocyte globulin, or immunoglobulin) within 1 year before the index date, or had been pregnant within 1 year before the index date.
Exposure and Outcome Measurements
Fluoroquinolones and penicillins were identified by the Anatomical Therapeutic Chemical classification codes: J01MA15, J01MA14, J01MA12 for fluoroquinolones, and J01CA, J01CE, J01CF, J01CR for penicillins. The primary outcome was 30-day pneumonia-related hospitalizations or ES visits, which were defined as an admission or an ES visit with a primary diagnosis of pneumonia.
Secondary outcomes included treatment failure and mortality during the 30 days after the index date. Treatment failure was defined as meeting any one of the following criteria: prolonged antibiotic use of 14 days or more, a second antibiotic added from a different class other than the index drug, and a change from oral antibiotics to injected medication. We considered the use of injected antibiotics to be an indicator of treatment failure because the guidelines of the NHI do not suggest initial intravenous antimicrobial medication at outpatient clinics. For most situations, injected antimicrobial medications are used for hospitalized patients as the treatment for serious infections due to the rapid systemic effect, or for patients with infections caused by antimicrobial-resistant microorganisms. Death was indicated by a discontinuation of the NHI for more than 6 months. Although individuals sometimes withdraw from the NHI for reasons other than death (eg, emigration, imprisonment), reasons other than death account for very few cases, given the single-payer health care system and the high coverage rate in Taiwan. This approach has been adopted by other studies conducted with the NHIRD data.22,23
Finally, we assessed the total medical costs and pneumonia-related costs within 30 days following the index date. Total medical costs were measured by summing up all of the medical costs occurring within 30 days after the index date, regardless of whether the costs were pneumonia-related or not. Pneumonia-related costs were calculated by summing up the 30-day total medical costs of inpatient or outpatient events with a primary diagnosis of pneumonia. All costs are presented in U.S. dollar values (1 USD ≒ 30 Taiwanese dollars).
Covariates
Baseline characteristics, including age, gender, comorbidities, medication, and health service utilization, were measured within 1 year before the index date. Baseline comorbid conditions measured in this study included respiratory diseases, cardiovascular diseases, and other comorbid conditions (Table 1). It should be noted that while we excluded patients receiving antituberculosis drugs within 30 days prior to the initiation of pneumonia treatment, whether a patient had a diagnosis of tuberculosis in the year preceding the pneumonia treatment was still included as a covariate in the regression analysis. We kept this indicator because tuberculosis often requires long-term treatment, and patients previously infected by tuberculosis may have decreased immunity and thus increased vulnerability to other infections. Preindex medication utilization was defined as any antibiotic use, disease-modifying antirheumatic drug/immunosuppressant use, or long-term corticosteroid use, defined as consecutive use of corticosteroids for at least 30 days during the 1-year preindex period. Last, we measured whether patients had pneumonia-related and other respiratory disease-related outpatient visits or hospitalizations and/or ES visits in the preindex period.
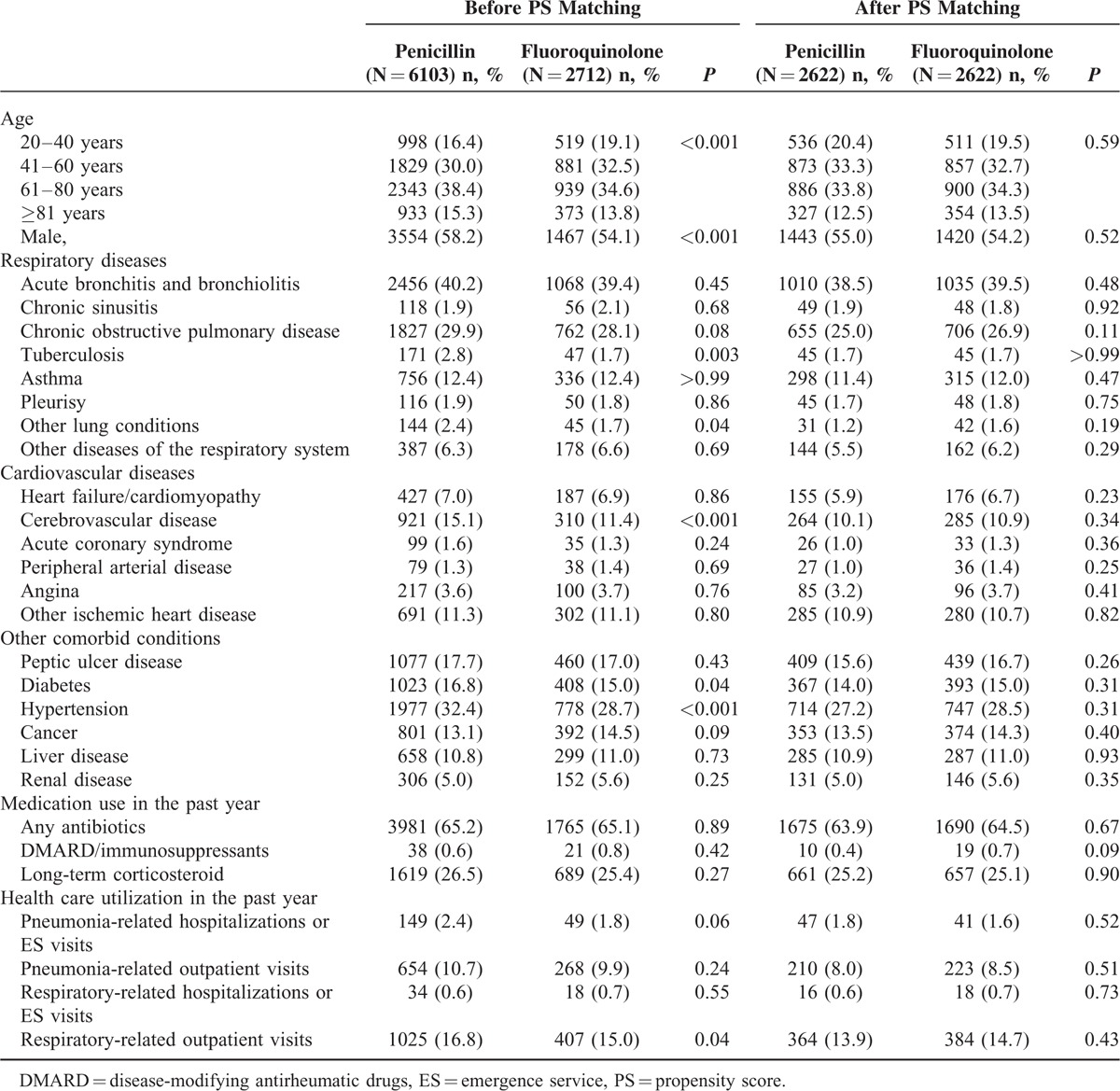
TABLE 1.
Baseline Characteristics of the Penicillin and Fluoroquinolone Groups Before and After Propensity Score Matching
Statistical Analysis
Student's t-tests and Chi-square tests were used to compare continuous and categorical variables, respectively. Propensity score (PS) matching was used to balance the baseline characteristics and thus control for potential confounding. We first generated the PSs using logistic regressions, which included the 31 potential predictors listed in the Covariates section. We next applied a greedy matching algorithm without trimming to create 1:1 pairs between the penicillin and the fluoroquinolone users. After the PS matching, Cox proportional hazard models were used to compare the rates of 30-day hospitalizations/ES visits and mortality between the 2 groups, and a logistic regression model was used to compare the likelihood of treatment failure within 30 days following the index date of the 2 groups. Treatment costs of the 2 groups were compared by median tests, since costs were not normally distributed. Several subgroup analyses were conducted. The effectiveness of fluoroquinolone-based therapy was compared to that of penicillin monotherapy and penicillin/macrolide combination therapy. We also stratified our analysis by age (age <65 and ≥65), since the elderly are at higher risk of CAP and often have worse CAP-related outcomes.24–26 Finally, the effectiveness of fluoroquinolone-based therapy was assessed among patients with asthma, chronic obstructive pulmonary disease, and acute bronchitis and bronchiolitis. Statistical analyses were performed in SAS software, Version 9.3.27
RESULTS
A total of 6103 penicillin-based and 2712 fluoroquinolone-based treatment episodes were identified, and 2622 matched-pair episodes were included in the final sample after PS matching (Figure 1). Table 1 shows the baseline characteristics of the 2 cohorts before and after the matching. Compared to patients who received penicillins, patients receiving fluoroquinolones tended to be younger; less likely to be males; less likely to have tuberculosis, other lung conditions, cerebrovascular diseases, diabetes, or hypertension; and less likely to have other respiratory disease-related outpatient visits before propensity matching. Approximately 6% (n = 353) of the pneumonia episodes were treated with a combination of penicillins and macrolides before the matching, and 4% (n�=�175), with penicillin and macrolide combination therapy after the matching (data not shown). All baseline characteristics were well balanced after the PS matching.
FIGURE 1.
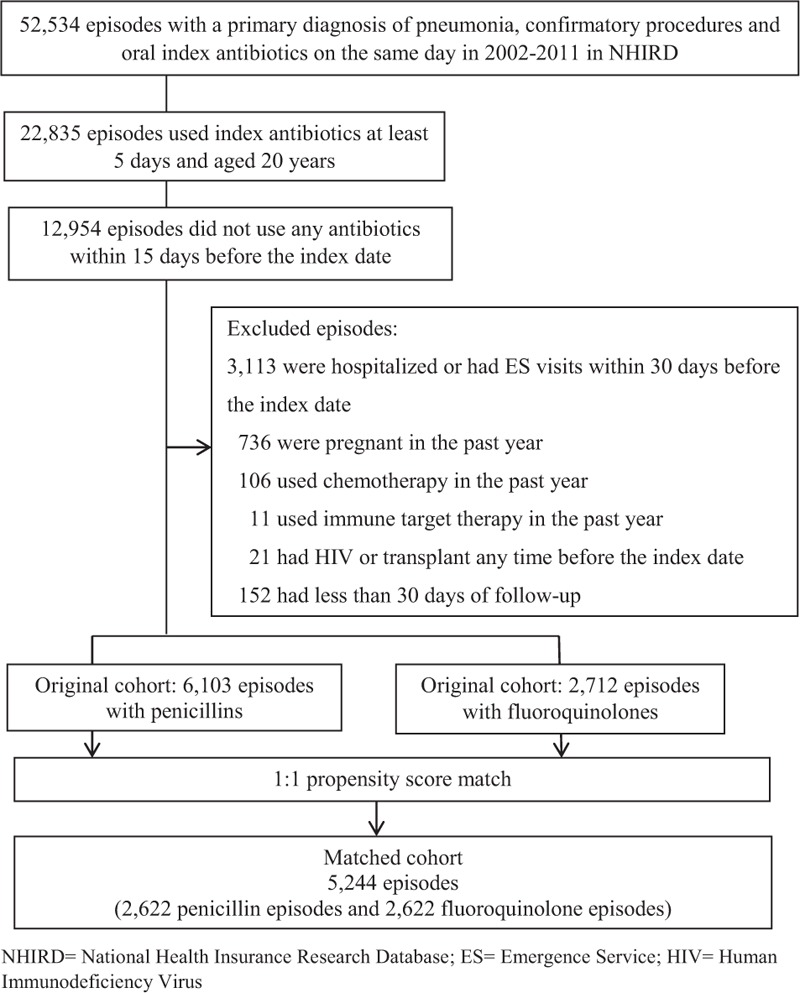
Sample size flow chart. ES = emergence service, HIV = human immunodeficiency virus, NHIRD = National Health Insurance Research Database.
Table 2 presents the results from the regression analysis of the matched sample. The adjusted results did not show any significant differences between fluoroquinolone-based and penicillin-based therapy (regardless of macrolide use) in 30-day hospitalization/ES visits or 30-day mortality in all adults aged ≥20 years old. We found that the odds of treatment failure of fluoroquinolone-based therapy were 12% lower than those of the penicillin-based therapy in all adults (adjusted odds ratio [AOR], 0.88; 95% confidence interval [CI], 0.77–0.99). Borderline lower odds of treatment failure were observed when we compared fluoroquinolone-based therapy to penicillin monotherapy (AOR, 0.91; 95% CI, 0.80–1.03) and to penicillin/macrolide combination therapy (AOR, 0.74; 95% CI, 0.53–1.04). These findings may have resulted from the reduced event numbers after the stratification. Finally, a significant reduction in the odds of 30-day mortality was observed among patients receiving fluoroquinolone versus penicillin monotherapy (adjusted hazard ratio, 0.42; 95% CI, 0.18–0.96).
TABLE 2.
Regression Results of the Primary and Secondary Outcomes for the Propensity Score-Matched Penicillin and Fluoroquinolone Groups
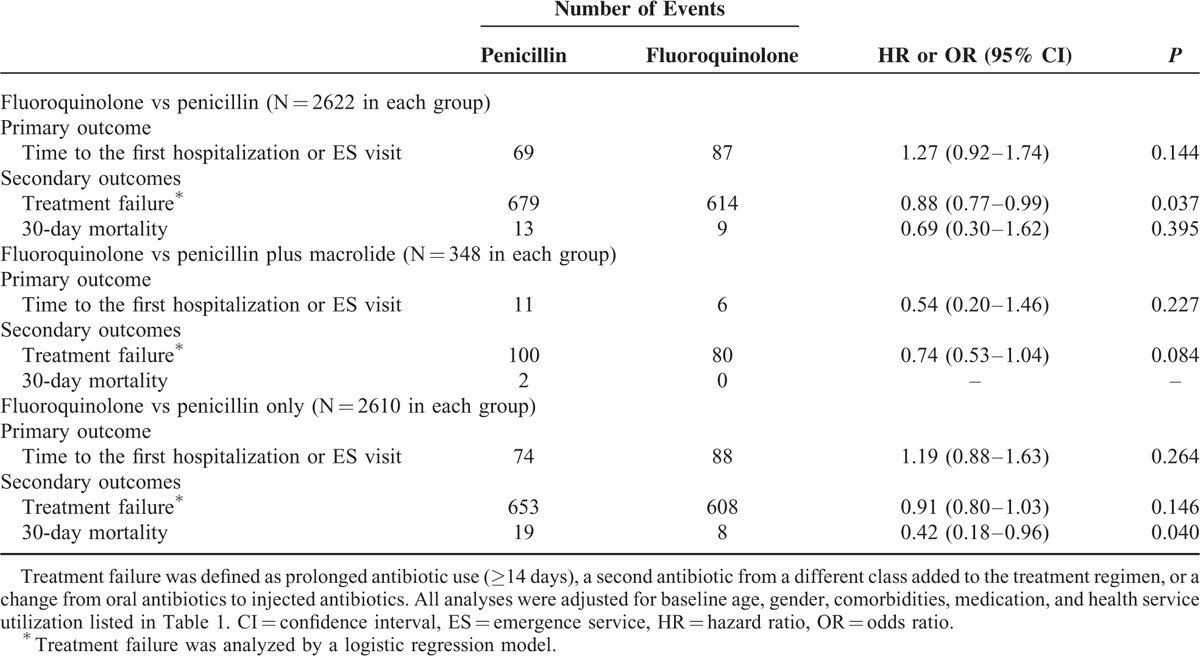
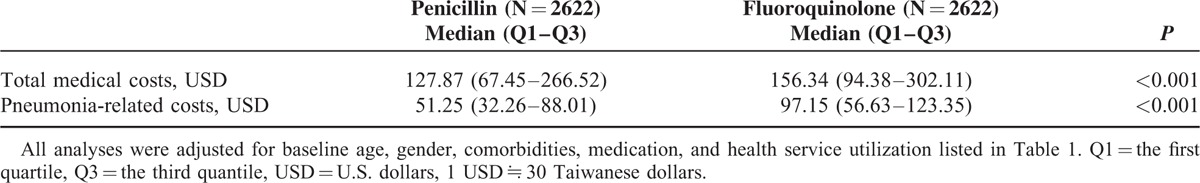
Table 3 shows the treatment costs for the matched cohort. The median for total medical costs for the penicillin-based and fluoroquinolone-base therapy groups were USD 127.87 (the first quantile–the third quantile [Q1–Q3], USD 67.45–266.52) and USD 156.34 (Q1–Q3, USD 94.38–302.11), respectively. Pneumonia-related costs for the penicillin-based and fluoroquinolone-base therapy groups were USD 51.25 (Q1–Q3, USD 32.26–88.01) and USD 97.15 (Q1–Q3, USD 56.63–123.35), respectively. The median tests suggested that both the total costs and the pneumonia-related costs were significantly lower in the penicillin-based than the fluoroquinolone-based therapy groups.
TABLE 3.
Total Medical Costs and Pneumonia-Related Costs Within 30 Days After the Index Date
Results of the subgroup analyses are shown in Figure 2. Similar to the main analysis, we did not find any significant associations between fluoroquinolone use and 30-day hospitalization/ES visits or 30-day mortality among patients aged ≥65 years old. A greater reduction in the odds of treatment failure was observed among the elderly receiving fluoroquinolones versus penicillins (AOR, 0.74; 95% CI 0.61–0.90), but no significant difference was found regarding the likelihood of treatment failure among patients aged less than 65 (AOR, 1.03; 95% CI 0.87–1.23). No significant differences were detected for either the primary or secondary outcomes in patients with asthma, chronic obstructive pulmonary disease, or acute bronchitis and bronchiolitis.
FIGURE 2.
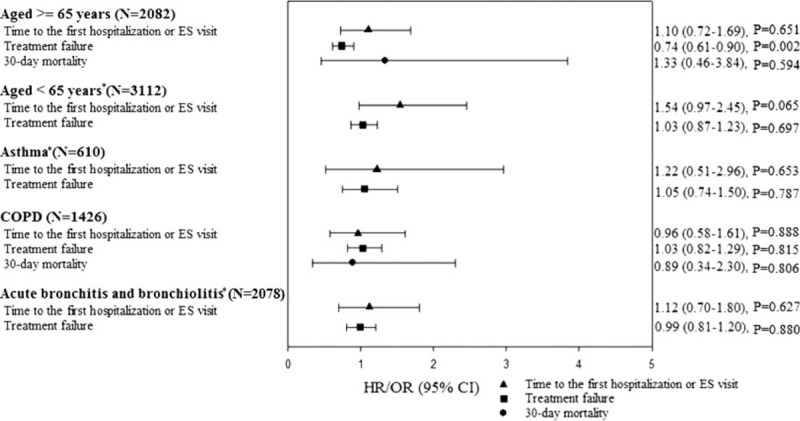
Adjusted results of subgroup analyses, by age and selected respiratory comorbidities. Results were generated from the propensity score matched samples. All analyses were adjusted for baseline age, gender, comorbidities, medication, and health service utilization listed in Table 1. ∗Analyses for 30-day mortality were not performed due to the low event numbers. CI = confidence interval, COPD = chronic obstructive pulmonary disease, ES = emergence service, HR = hazard ratio, OR = odds ratio.
DISCUSSION
To our knowledge, this is the first study to compare the effectiveness of fluoroquinolones and penicillins for CAP treatment on an outpatient basis in an Asian population. Our results suggest that patients who initiated fluoroquinolones were less likely than patients initiating penicillins to experience treatment failure, but no differences were found regarding 30-day hospitalizations/ES visits or mortality. These findings are consistent with those of a previous study showing that the likelihood of treatment failure tended to be lower after initial treatment with levofloxacin than after initial treatment with macrolides.17 All these results combined suggest that fluoroquinolones may be an effective empirical treatment for CAP in outpatient settings.
The potential effectiveness of fluoroquinolones-based therapy may be explained by the antimicrobial susceptibility of S. pneumoniae isolates in this area. Depending on the isolates and testing criteria, the nonsusceptibility rates of penicillins for nonmeningitis isolates ranged from 58%–72% in 2000–2003 to 16%–30% in 2007–2008, as compared to the 4% t 10% nonsusceptibility rates of fluoroquinolones in 2002 to 2009.28–30 In addition, resistance through plasmid-mediated production of β-lactamase is common for Haemophilus influenzae (near 12% of CAP), which is usually susceptible to fluoroquinolones.9 Last, fluoroquinolones also have the advantage of covering atypical pathogens, which may cause 30% or more of the cases of pneumonia. The relatively lower resistance rate and broader spectrum of fluoroquinolones may have led to the lower rate of treatment failure rate in this study. However, the reduction in the odds of treatment failure may have been marginal, since the upper limit of the 95% CI was very close to one for all adults aged ≥20 (AOR, 0.88; 95% CI 0.77–0.99).
Although the effect of the reduced risk of treatment failure appeared to be marginal in the all adults group, this effect seemed to be more evident among the elderly (AOR, 0.74; 95% CI 0.61–0.90). Given that the elderly generally have more comorbidities and are at a higher risk of drug-resistant microorganism infections and Legionnaire disease, they should be considered for fluoroquinolone-based therapy.19 Although no clear conclusion has been reached, some evidence suggests that respiratory fluoroquinolones may be more effective than a combination of β-lactam and macrolide therapy in the elderly.24,25 The once-daily schedule of the fluoroquinolone-based therapy may also facilitate the treatment of the elderly.
Consistent with previous findings,17 we did not find any differences in hospitalization/ES visit rate and mortality rate within 30 days after the initial antibiotic treatment between the 2 groups. Nevertheless, in the stratified analysis comparing fluoroquinolones to penicillin monotherapy, our results showed a significant rate reduction in 30-day mortality for all adults (adjusted hazard ratio, 0.42; 95% CI, 0.18–0.96; P-value 0.040). This result may be explained by the higher potency and broader spectrum as well as the lower rate of resistance of fluoroquinolones.7,8,28–30
Higher medical costs and pneumonia-related costs were observed in the fluoroquinolone group than in the penicillin group. Even though we found a high percentage (70%) of the use of a high-cost penicillin product, amoxicillin/clavulanate (Augmentin), in the penicillin group, the average cost of fluoroquinolones was higher than that of penicillins (USD 2.91 vs USD 1.27 per tablet), which may partially explain the high costs associated with the fluoroquinolone-based therapy. Other potential explanations include that low proportion of macrolide combination in the penicillin group and patients receiving fluoroquinolones may receive costly intensive treatment, since they often have more severe pneumonia and other comorbidities.
This study has several limitations. First, an outpatient diagnosis of pneumonia may lack some degree of validity, since the signs and symptoms of pneumonia overlap with several other respiratory diseases. To improve the validity of the pneumonia measure, we required that patients have not only a primary diagnosis of pneumonia but also a diagnostic procedure for pneumonia, and that they be treated with antibiotics for at least 5 days in order to qualify as having a pneumonia episode. Second, we were unable to assess disease severity on the basis of claims information. We attempted to solve this problem and composed a homogeneous group through careful control: restricting our study sample with specific criteria, controlling for several respiratory and cardiovascular comorbidities, and controlling for medications that may influence the risk of being infected in the analysis. In addition, we were unable to further stratify our analysis based on clinical and/or pathological features or identify potential adverse events associated with the antimicrobial therapy, such as abnormalities of renal and liver functional tests, because the laboratory test results were not available in the NHIRD. Given the low number of events, we may not have been able to detect the differences in the subgroup and stratified analyses. Finally, the claims data provided only information on prescription refills, and none on actual compliance with treatment. However, unless the compliance differed significantly between the penicillin-based and fluoroquinolone-based therapy groups, the problem of nonadherence should not bias our results other than attenuating the results toward the null.
CONCLUSION
In conclusion, our results suggest that the likelihood of treatment failure of fluoroquinolone-based therapy is lower than that of penicillin-based therapy for CAP. However, this effect may be marginal. Further investigations comparing the effectiveness of these 2 treatment options are warranted.
Acknowledgments
A portion of the study results has been accepted as a poster presentation in the 2015 APhA Annual Meeting and Exposition.
The study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent the views of the Bureau of National Health Insurance, the Department of Health, or the National Health Research Institutes.
Footnotes
Abbreviations: AOR = adjusted odds ratio, CAP = community-acquired pneumonia, CI = confidence interval, ES = emergence service, LHID = Longitudinal Health Insurance Database, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, PS = propensity score, Q1 = The First Quartile, Q3 = The Third Quartile, USD = U.S. Dollars.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 1996; 275:134–141. [PubMed] [Google Scholar]
- 2.Infectious Diseases Society of Taiwan. Guidelines on antimicrobial therapy of pneumonia in adults in Taiwan, revised 2006. J Microbiol Immunol Infect 2007; 40:279–283. [PubMed] [Google Scholar]
- 3.Feldman C, Anderson R. Antibiotic resistance of pathogens causing community-acquired pneumonia. Semin Respir Crit Care Med 2012; 33:232–243. [DOI] [PubMed] [Google Scholar]
- 4.Felmingham D. Comparative antimicrobial susceptibility of respiratory tract pathogens. Chemotherapy 2004; 50 Suppl 1:3–10. [DOI] [PubMed] [Google Scholar]
- 5.Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents 2010; 36:197–204. [DOI] [PubMed] [Google Scholar]
- 6.Low DE. What is the relevance of antimicrobial resistance on the outcome of community-acquired pneumonia caused by Streptococcus pneumoniae? (should macrolide monotherapy be used for mild pneumonia?). Infect Dis Clin North Am 2013; 27:87–97. [DOI] [PubMed] [Google Scholar]
- 7.Jones RN, Mandell LA. Fluoroquinolones for the treatment of outpatient community-acquired pneumonia. Diagn Microbiol Infect Dis 2002; 44:69–76. [DOI] [PubMed] [Google Scholar]
- 8.Vardakas KZ, Siempos II, Grammatikos A, et al. Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials. CMAJ: Can Med Assoc J 2008; 179:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman C, Anderson R. Bacteraemic pneumococcal pneumonia: current therapeutic options. Drugs 2011; 71:131–153. [DOI] [PubMed] [Google Scholar]
- 10.Frei CR, Jaso TC, Mortensen EM, et al. Medical resource utilization among community-acquired pneumonia patients initially treated with levofloxacin 750 mg daily versus ceftriaxone 1000 mg plus azithromycin 500 mg daily: a US-based study. Curr Med Res Opin 2009; 25:859–868. [DOI] [PubMed] [Google Scholar]
- 11.Gleason PP, Meehan TP, Fine JM, et al. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med 1999; 159:2562–2572. [DOI] [PubMed] [Google Scholar]
- 12.Lodise TP, Kwa A, Cosler L, et al. Comparison of beta-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother 2007; 51:3977–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portier H, Brambilla C, Garre M, et al. Moxifloxacin monotherapy compared to amoxicillin-clavulanate plus roxithromycin for nonsevere community-acquired pneumonia in adults with risk factors. Eur J Clin Microbiol Infect Dis 2005; 24:367–376. [DOI] [PubMed] [Google Scholar]
- 14.Querol-Ribelles JM, Tenias JM, Querol-Borras JM, et al. Levofloxacin versus ceftriaxone plus clarithromycin in the treatment of adults with community-acquired pneumonia requiring hospitalization. Int J Antimicrob Agents 2005; 25:75–83. [DOI] [PubMed] [Google Scholar]
- 15.Ruhe J, Mildvan D. Does empirical therapy with a fluoroquinolone or the combination of a beta-lactam plus a macrolide result in better outcomes for patients admitted to the general ward? Infect Dis Clin North Am 2013; 27:115–132. [DOI] [PubMed] [Google Scholar]
- 16.Weiss K, Tillotson GS. The controversy of combination vs monotherapy in the treatment of hospitalized community-acquired pneumonia. Chest 2005; 128:940–946. [DOI] [PubMed] [Google Scholar]
- 17.Ye X, Sikirica V, Schein JR, et al. Treatment failure rates and health care utilization and costs among patients with community-acquired pneumonia treated with levofloxacin or macrolides in an outpatient setting: a retrospective claims database analysis. Clin Ther 2008; 30:358–371. [DOI] [PubMed] [Google Scholar]
- 18.Heffelfinger JD, Dowell SF, Jorgensen JH, et al. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch Intern Med 2000; 160:1399–1408. [DOI] [PubMed] [Google Scholar]
- 19.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44 Suppl 2:S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDougall C, Guglielmo BJ, Maselli J, et al. Antimicrobial drug prescribing for pneumonia in ambulatory care. Emerg Infect Dis 2005; 11:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Health Insurance Research Database. http://nhird.nhri.org.tw/en/index.htm [Accessed December 10, 2013]. [Google Scholar]
- 22.Huang ST, Lin CL, Chang YJ, et al. Pneumococcal pneumonia infection is associated with end-stage renal disease in adult hospitalized patients. Kid Int 2014; 86:1023–1030. [DOI] [PubMed] [Google Scholar]
- 23.Wu C-Y, Chen Y-J, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012; 308:1906. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez F, Masia M. Improving outcomes of elderly patients with community-acquired pneumonia. Drugs Aging 2008; 25:585–610. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman D. Community-acquired pneumonia in the elderly: a practical guide to treatment. Drugs Aging 2000; 17:93–105. [DOI] [PubMed] [Google Scholar]
- 26.Simonetti AF, Viasus D, Garcia-Vidal C, et al. Management of community-acquired pneumonia in older adults. Ther Adv Infect dis 2014; 2:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SAS Institute Inc. SAS/STAT 9.3® User's Guide. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 28.Kim SH, Song JH, Chung DR, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother 2012; 56:1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su LH, Wu TL, Kuo AJ, et al. Antimicrobial susceptibility of Streptococcus pneumoniae at a university hospital in Taiwan, 2000–07: impact of modified non-meningeal penicillin breakpoints in CLSI M100-S18. J Antimicrob Chemother 2009; 64:336–342. [DOI] [PubMed] [Google Scholar]
- 30.Tsai HY, Lauderdale TL, Wang JT, et al. Updated antibiotic resistance and clinical spectrum of infections caused by Streptococcus pneumoniae in Taiwan: emphasis on risk factors for penicillin nonsusceptibilities. J Microbiol Immunol Infect 2013; 46:345–351. [DOI] [PubMed] [Google Scholar]


