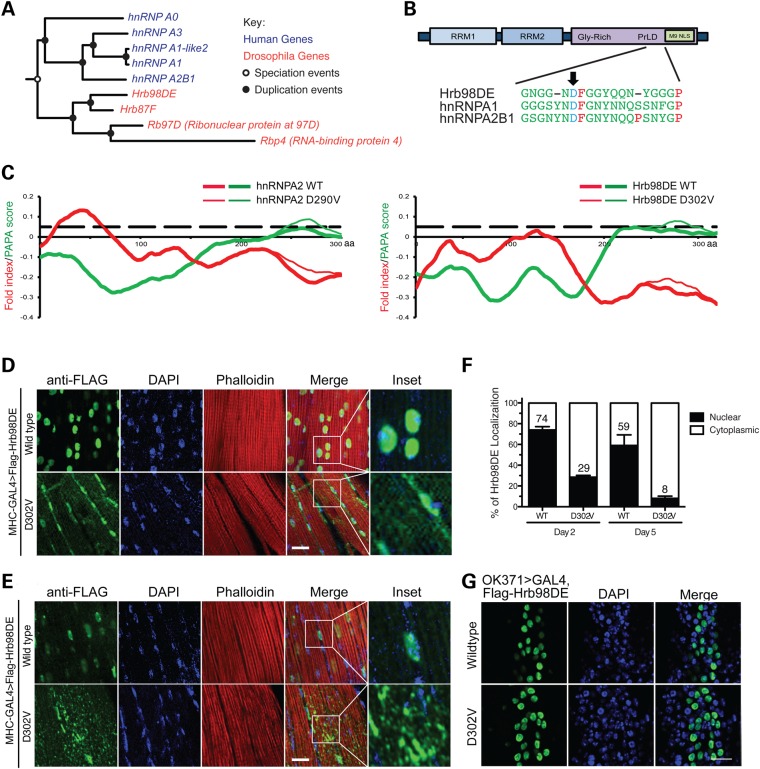
Figure 1.
Ectopic expression of Hrb98DE D302V in Drosophila muscle induces age-related accumulation of cytoplasmic aggregates. (A) Phylogenetic tree of the orthologic/paralogic relationships among genes of human and Drosophila HNRNPA2B1 family members. (B) Schematics show conserved domains in Hrb98DE and its amino acid sequence alignment with hnRNPA1 and hnRNPA2B1. Arrow shows the disease-related mutations, which are D290V in hnRNPA2 (dominant isoform of hnRNPA2B1) and D262V in hnRNPA1, and their corresponding mutation D302V in Hrb98DE. RRM, RNA recognition motif; Gly-rich, glycine-rich domain; PrLD, prion-like domain; M9 NLS, M9 nuclear localization signal. (C) The mutation significantly enhances the predicted prion propensity of hnRNPA2 and Hrb98DE. PAPA scores (green) and fold indexes (red) were calculated by using the PAPA algorithm, and those of WT and D302V mutant Hrb98DE were compared. Black dashed line indicates the prion propensity cutoff of the PAPA algorithm, which is 0.05. (D) Cellular distribution of Flag-Hrb98DE WT and D302V in day 2 adult Drosophila indirect flight muscle. Both WT and D302V Flag-Hrb98DE (green) co-localize with DAPI-stained nuclei (blue). (E) Cellular distribution of Flag-Hrb98DE WT and D302V in day 5 adult Drosophila indirect flight muscle. (F) Quantitative analysis of cellular distribution of Flag-Hrb98DE WT and D302V in day 2 and 5 Drosophila. The signal of either WT or D302V Flag-Hrb98DE was referred to DAPI to determine its nuclear localization. The percentages of WT and D302V Flag-Hrb98DE in nuclei (filled bar) or cytoplasm (opened bar) are shown in the bar graph. Scale bar represents 20 µm. (G) Immunofluorescence shows nuclear distribution of WT or D302V Flag-Hrb98DE in motor neurons under the control of the OK371>GAL4 promoter.