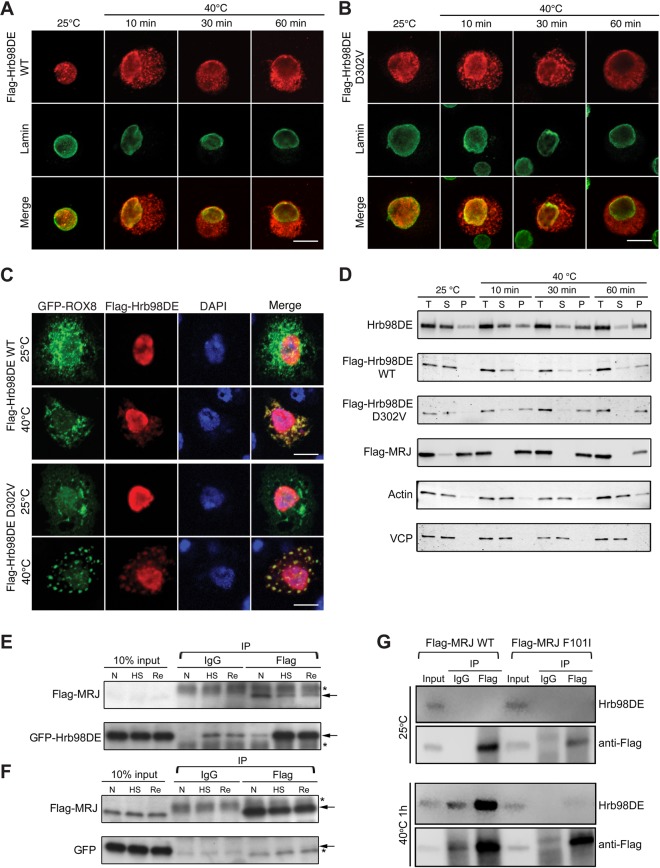
Figure 7.
Heat shock changed the solubility and cellular localization of Hrb98DE and induced interaction between Hrb98DE and MRJ. (A) S2 cells transfected with Flag-Hrb98DE WT were fixed before or after 40°C heat shock and stained with anti-Flag (red) and anti-lamin (green, nuclear marker) to determine the cellular localization of Flag-Hrb98DE. Flag-Hrb98DE WT resided in the nucleus before heat shock and quickly translocated to the cytoplasm after heat shock. (B) Same experiment as (A), except Flag-Hrb98DE D302V was transfected into S2 cells. (C) S2 cells transfected with Flag-Hrb98DE and ROX8-GFP were treated with or without heat shock. Flag-Hrb98DE co-localized with ROX8-GFP in the cytoplasm after heat shock treatment. (D) Heat shock-induced changes in Hrb98DE's solubility in 0.2% Triton X-100 lysis buffer (Supplementary Material, Fig. S7). Equal amounts of cells were lysed either in Laemmli sample buffer to obtain a total (T) sample or in lysis buffer to obtain the soluble fraction (S) and pellet fraction (P). Endogenous Hrb98DE or transfected Flag-Hrb98DE WT, D302V or Flag-MRJ was detected by western blotting to determine their solubility to lysis buffer. Hrb98DE was detected in the soluble fraction (S) at normal condition. After heat shock, Hrb98DE became insoluble to lysis buffer and became detectable in the pellet fraction (P). The solubility of VCP and actin was not changed after heat shock and used as loading controls along with Sypro Ruby-stained gel (Supplementary Material, Fig. S8C). (E) Cells transfected with Flag-MRJ and GFP-Hrb98DE were treated at normal (25°C, N), heat shock (40°C for 1 h, HS) or recovery (30 min at 25°C after 1 h 40°C treatment and recovery). Using magnetic bead-based co-IP, GFP-Hrb98DE was found to be increasingly associated with Flag-MRJ in the heat shock (HS) and recovery (Re) conditions compared with that in normal (N) condition. In control IgG co-IP, only trace amounts of GFP-Hrb98DE are detected after heat shock, which may be due to the contamination of the insoluble GFP-Hrb98DE. (F) As a control, cells were transfected with Flag-MRJ and GFP and co-IP was performed as described in (E). GFP was not found to co-precipitate with Flag-MRJ in any condition. (G) Cells that transfected with either WT or F101I mutant form of Flag-MRJ were treated with heat shock, and co-IP was performed as described in (E) and (F). Endogenous Hrb98DE proteins were detected using a monoclonal antibody we generated (Supplementary Material, Fig. S4). Arrows in (E) and (F), band of interest. Asterisks in (E) and (F), nonspecific band. Scale bars, 10 µm.