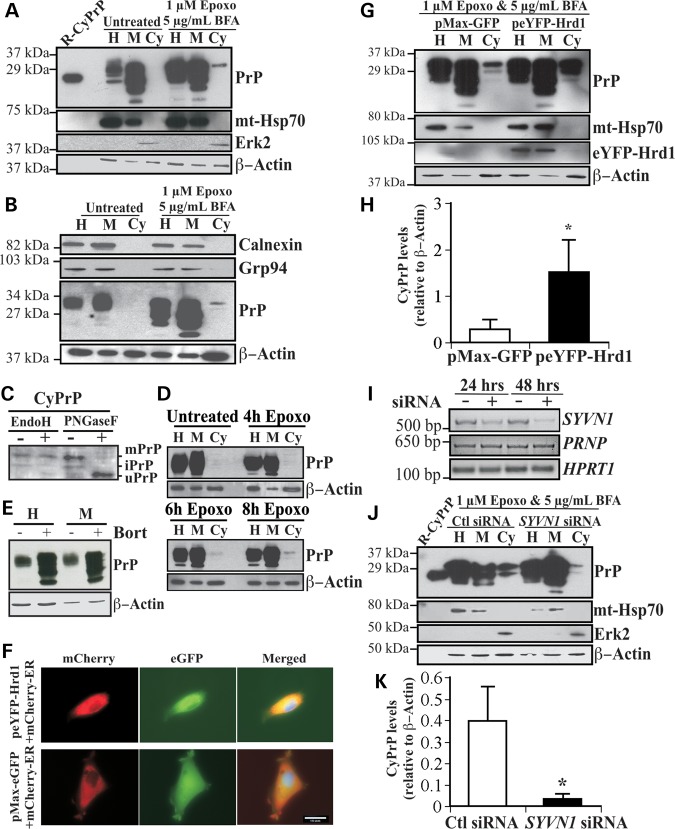
Figure 1.
Overexpression of Hrd1 enhances PrP retrotranslocation while target knockdown by siRNA abolishes CyPrP in CR7 cells. (A) Western blot analysis with anti-PrP (3F4), anti-mitochondrial heat shock protein 70 (mt-Hsp70), anti-Erk2 and anti-β-Actin in CR7 cells treated for 18 h with epoxomicin (epoxo) and brefeldin A (BFA) and fractionated into homogenate (H), membrane (M) and cytosolic (Cy) fractions. Equal amounts of proteins from matched fraction pairs were loaded (example: Cy versus Cy). R-PrP; recombinant cytosolic PrP23–231. (B) Western blot with anti-calnexin, Grp94, PrP and actin of the subcellular fractions. (C) Western blot analyses of Endo H and PNGase F treated CyPrP with the 3F4 anti-PrP antibody show mature PrP (mPrP), immature PrP (iPrP) and unglycosylated PrP (uPrP). The small bar left to the panel represents the 27 kDa molecular weight marker. (D) Western blot analyses with anti-PrP 3F4 of proteins from subcellular fractionated CR7 cells treated with epoxomicin for 0, 4, 6 and 8 h. (E) Western blot analyses of the whole homogenate (H) or membrane fraction (M) of proteins from CR7 cells without or with bortezomib. (F) Fluorescent light microscopy of CR7 cells co-transfected with mCherry-ER and peYFP-Hrd1 or GFP mammalian expression vector. Nuclear DNA was stained with Hoechst 33342 staining. Scale bar represents 200 μm. (G) Western blot analysis with anti-PrP, anti-mt-Hsp70, anti-Hrd1, anti-Erk2 and anti-β-actin in CR7 cells transfected with either pMaxGFP or peYFP-Hrd1 and subjected to the subcellular fractionation. Proteins were loaded as in Figure 1A. (H) Relative CyPrP to membrane PrP levels evaluated by densitometry and depicted as fold increase over β-actin protein levels. (I) Ethidium bromide agarose gel of SYVN1, PRNP and HPRT1 amplicons obtained by RT-PCR from CR7 cells transfected for 24 or 48 h with either scrambled non-targeting control or SYVN1-targeting siRNA. (J) Western blot analysis with anti-PrP, anti-mt-Hsp70, anti-Erk2 and anti-β-actin in proteins extracted from subcellular fractions of CR7 cells transfected with 250 nm of either non-targeting scrambled or SYVN1-targeting siRNA followed by 18 h treatment with 1 µM epoxomicin and 5 µg/ml BFA. Proteins are loaded as in Figure 1A. (K) PrP in the cytosolic fraction relative to PrP in the membrane fraction of CR7 cells transfected with either non-targeting scrambled or SYVN1-targeting siRNA. CyPrP levels, evaluated by densitometry, are depicted as fold increase over β-actin. All graphed data represent the mean ± SEM of at least three independent experiments and were analyzed by independent samples t-test, *P < 0.05.