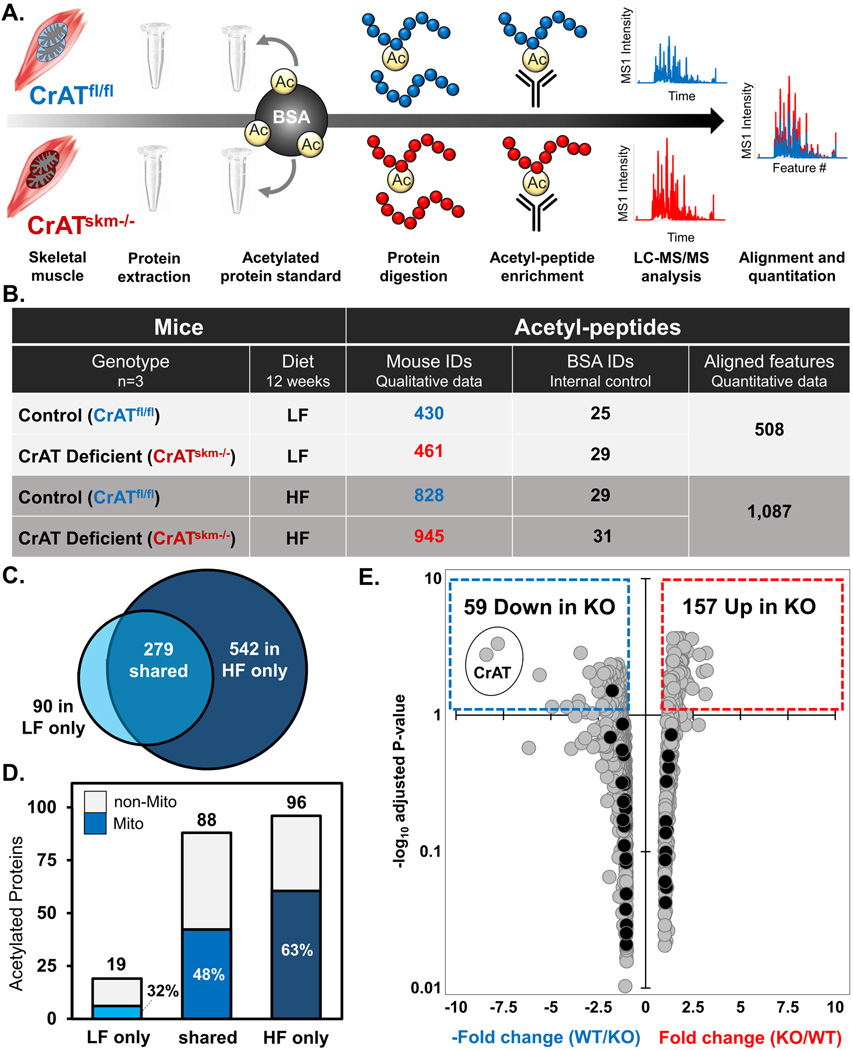
Figure 2. High fat feeding expands the detectable acetyl-proteome in skeletal muscle.
(a) Schematic of the experimental workflow. After 12 weeks of feeding either a low fat (LF) or high fat (HF) diet, mouse quadriceps muscles were excised and protein extracted. Acetylated BSA standard was added to each sample prior to protein digestion. Peptides were immunoprecipitated (IP) with anti-acetyl lysine antibody and acetylpeptides were analyzed using high resolution LC-MS/MS to evaluate the acetylpeptide inventory and perform label-free quantitation. (b) Qualitative summary and data metrics for acetylpeptides identified at 1% FDR in quadriceps muscles from CrATskm−/− and CrATfl/fl mice. (c) Venn diagram showing overlap between non-redundant sites of acetylation mapped by acetylpeptides identified at 1% FDR in quadriceps muscles from CrATfl/fl mice fed a LF compared to HF diet. (d) Corresponding bar graph indicating the number of skeletal muscle proteins from CrATfl/fl mice containing at least one acetylation site, with the dark-shaded region indicating the fraction localizing to the mitochondrion. (e) Volcano plot of fold-change vs. significance of quantified acetylpeptides in CrATskm−/− compared to CrATfl/f mice fed a HF diet. The x-axis indicates magnitude (arithmetic scale) and directionality (negative numbers indicate a decrease in KO) of abundance changes and the y-axis shows the adjusted P-values in −log10 space. Significantly down-regulated and up-regulated peptides (adjusted P<0.05, or −log10 adjusted P>1.3) in CrATskm−/− quadriceps are contained within the dotted boxes in blue and red, respectively.