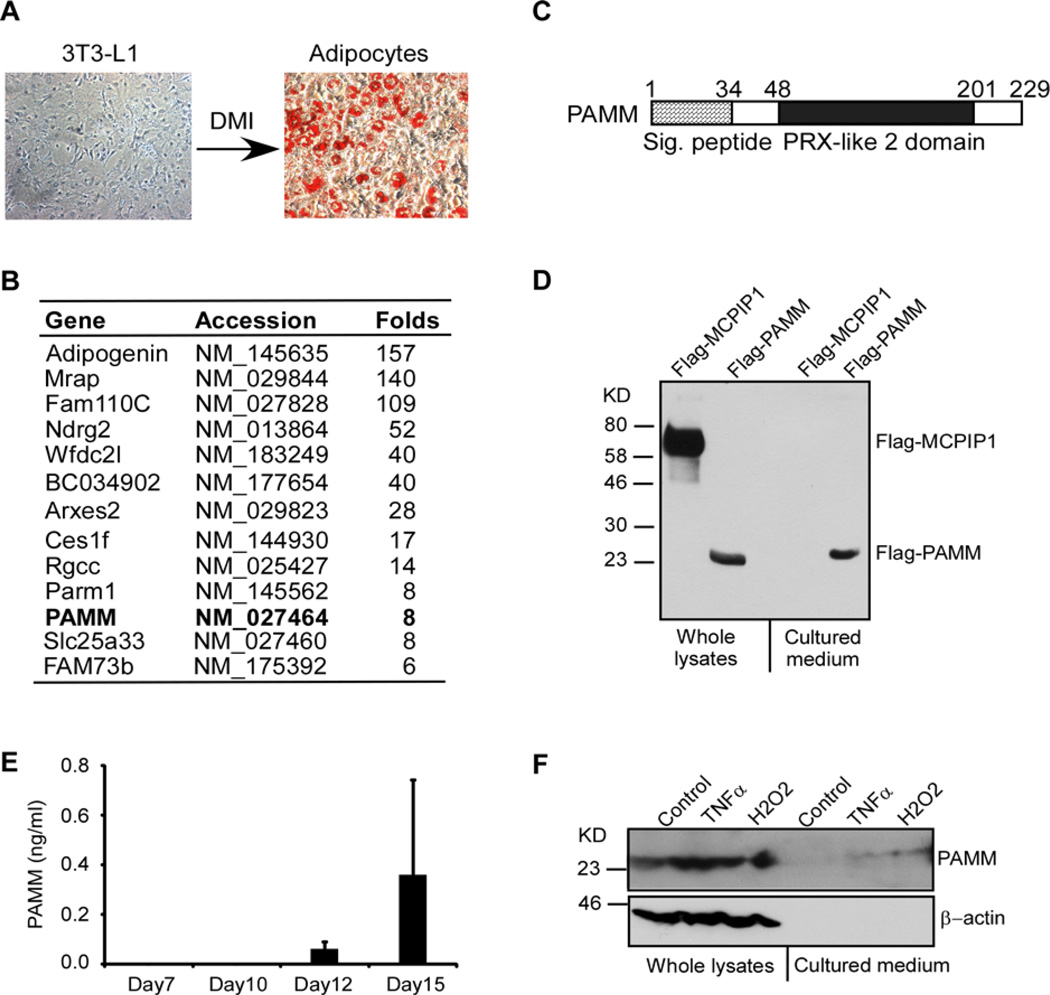
Figure 1. Identification of PAMM as a secreted protein from adipocytes.
(A) 2-day post-confluent 3T3-L1 cells were incubated with a cocktail of DMI for 10 days to induce differentiation into adipocytes. The cells were stained by Oil O and the images were taken by Nikon microscope system. (B) Affymatrix microarray analysis of gene expression in 3T3-L1 cells and adipocytes. Uncharacterized genes that were up-regulated by more than 5-fold in adipocytes and encoded proteins containing putative signal peptides are listed in this table. (C) Scheme of human PAMM protein structure. Human PAMM is a 229 amino acid protein containing a putative signal peptide and a PRX-like 2 domain. (D) HEK293 cells were transiently transfected with Flag-PAMM or Flag-MCPIP1. Whole cell lysates and culture medium were analysed for Flag-PAMM and Flag-MCPIP1 by Western blot analysis with anti-Flag antibody. The results are representative of three independent experiments, which showed similar results. (E) Human primary preadipocytes were incubated with adipocyte differentiation medium for different days as indicated. The cultured media were collected and concentrated for ELISA analysis of PAMM protein. Data are presented as mean ± S.D. (F) Raw264.7 cells were stimulated with or without 10 ng/ml TNFα or 400 µM H2O2 for 4 h. Whole cell lysates and culture medium were collected for Western blot analysis with PAMM and β-actin antibodies.