Abstract
Pluripotent or multipotent cell-based therapeutics are vital for skeletal reconstruction in non-healing critical-sized defects since the local endogenous progenitor cells are not often adequate to restore tissue continuity or function. However, currently available cell-based regenerative strategies are hindered by numerous obstacles including inadequate cell availability, painful and invasive cell-harvesting procedures, and tumorigenesis. Previously, we established a novel platform technology for inducing a quiescent stem cell-like stage using only a single extracellular proteoglycan, fibromodulin (FMOD), circumventing gene transduction. In this study, we further purified and significantly increased the reprogramming rate of the yield multipotent FMOD reprogrammed (FReP) cells. We also exposed the ‘molecular blueprint’ of FReP cell osteogenic differentiation by gene profiling. Radiographic analysis showed that implantation of FReP cells into a critical-sized SCID mouse calvarial defect, contributed to the robust osteogenic capability of FReP cells in a challenging clinically relevant traumatic scenario in vivo. The persistence, engraftment, and osteogenesis of transplanted FReP cells without tumorigenesis in vivo were confirmed by histological and immunohistochemical staining. Taken together, we have provided an extended potency, safety, and molecular profile of FReP cell-based bone regeneration. Therefore, FReP cells present a high potential for cellular and gene therapy products for bone regeneration.
Keywords: Fibromodulin (FMOD), Reprogramming, Fibromodulin reprogrammed (FReP) cells, Differentiation, Osteogenesis
1. Introduction
Although bone tissue has a comparatively high regenerative capacity, its self-repairing process fails in critical-sized defects due to a lack of sufficient local osteoprogenitors to restore tissue continuity or function [1, 2]. Unfortunately, isolation or generation of safer and readily available regenerative cell sources remains a major challenge to date. For instance, direct transplantation of committed osteoblasts is hindered by inadequate cell availability, limited cell spreading, and poor survivability of implanted cells [3]. Meanwhile, invasive harvesting procedures reduce the benefits of mesenchymal stem cell (MSC) usage [4–6], whereas the risk of tumorigenesis hinders the clinical application of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) [7–12]. Therefore, an urgent need exists to establish an alternative regenerative cell source for bone regeneration without significant tumorigenic risk.
To conquer this obstacle, we previously reported a novel technology that directly reprogrammed human dermal fibroblasts into a multipotent stage by using a single extracellular matrix proteoglycan, fibromodulin (FMOD), which is broadly distributed in connective tissues and has been found to be a critical component for maintenance of endogenous stem cells niches by modulating the bioactivities of growth factors [13, 14]. FMOD ReProgrammed (FReP) cells, the outcome of the FMOD reprogramming, expressed pluripotency markers, established embryoid bodies, and presented the capability to differentiate into ectoderm, mesoderm, and endoderm derivatives in vitro [15]. Moreover, transplanting in vitro pre-osteogenic initiated FReP cells in the muscle pouch of severe combined immunodeficiency (SCID) mouse led to bone generation without tumor formation [15], which suggested that FReP cells could be used as a novel osteoprogenitor for bone regeneration.
In the current study, we further improved the FMOD reprogramming technology. In addition, to further assess the potential of FReP cells in bone regeneration, we profiled the gene expression of FReP cells during osteogenesis in vitro and evaluated the in vivo osteogenic efficacy of FReP cells in a clinically relevant critical-sized calvarial defect model.
2. Materials and Methods
2.1. FMOD production
cDNA of human FMOD transcript (Genbank assessor number: NM_002023) was subcloned into a commercially available vector pSecTag2A (Life Technology, Grand Island, NY) with C-terminal His-tag and transfected into CHO-K1 cells (ATCC, Manassas, VA) [16]. After establishing a stable expression clone, the FMOD was produced and purified by a contract research organization GenScript (Piscataway, NJ). Briefly, stable human recombinant FMOD-expressing CHO-K1 cell line was cultured in 1L serum-free Freestyle CHO Expression Medium (Invitrogen) at 37°C, 5% CO2 in an Erlenmeryer flask. Cell culture supernatant was harvested on day 10 for purification with HiTrap™ IMAC HP, 1-mL column (GE Healthcare, Uppsala, Sweden). The fractions from a 100 mM imidazole elution were collected and dialyzed against 20 mM phosphate-buffered saline (PBS), pH 7.4. After that, the sample with low conductivity was loaded onto HiTrap™Q HP 1-mL column (GE Healthcare) for further purification. The purified protein was then evaluated by SDS-PAGE and Western blot (Supplementary Fig. 1). FMOD purified under non-reducing conditions was dialyzed again and sterilized for cell reprogramming.
2.2. Cell Culture
Human newborn foreskin BJ-fibroblasts (ATCC) were cultured in a 4:1 mixture of Dulbecco’s Modified Eagle’s Medium (containing 4 mM L-glutamine, 1.0 g/L glucose and 1.5 g/L sodium bicarbonate; Life Technology) and Medium 199 (Life Technology), supplemented with 10% fetal bovine serum (FBS; Life Technology) and 1% penicillin/streptomycin (P/S; Life Technology). BJ-fibroblast-derived iPSCs (BJ-iPSCs) obtained by conventional retrovirus-mediated method [17] were maintained on Matrigel™ hESC-qualified Matrix (BD Biosciences, San Jose, CA) pre-coated plates with mTESR®1 medium (STEMCELL Technologies, Vancouver, Canada).
2.3. FMOD reprogramming
4 × 105 cells/well BJ-fibroblasts were seeded in 6-well culture plates overnight to confluence before exposure to 0.4 mg/ml recombinant human FMOD in DMEM medium supplemented with 1% PS for reprogramming under a serum-free condition. Fresh medium was changed daily [15]. After 21-day continual FMOD reprogramming, FReP cells were harvested with ReLeSR™ (an enzyme-free hESC and hiPSC selection and passaging reagent [18, 19]; STEMCELL Technologies), and cultured on Matrigel™ hESC-qualified Matrix coated-plates with mTESR®1 medium [20].
2.4. Embryoid body (EB) formation and characterization
FReP cells harvested by ReLeSR™ reagent were seeded on AggreWell™ 800 Plates with AggreWell™ medium (STEMCELL Technologies) for EB formation following the manufacturer’s instruction. After 3 days, EBs were harvested and cryostat sectioned at 5 µm for immunological staining.
2.5. In vitro differentiation towards endoderm derivatives
FReP cells harvested by ReLeSR™ reagent were cultivated in RPMI 1640 medium (Life Technology) supplied with 2% FBS, 2 mM L-glutamine, 1% P/S, and 100 ng/ml recombinant activin A (R&D systems, Minneapolis, MN) for 4 days, and then cultured without activin A for an additional 8 days [15].
2.6. In vitro osteogenic differentiation
For in vitro osteogenesis, FReP cells and their parental BJ-fibroblasts were transferred to AF solution (Life Technology) pre-coated plates and cultured in osteogenic medium [α-Modified Eagle’s Medium (Life Technology) supplied with 10% FBS, 50 µg/ml ascorbic acid (Sigma-Aldrich, St. Louis, MO), 10 mM β-glycerophosphate (Sigma-Aldrich), 10−8 M dexamethasone (Sigma-Aldrich)and 1% P/S] for 4 weeks[15].
2.7. Animal model
All animal surgeries were performed under institutional approved protocols provided by Chancellor’s Animal Research Committee at UCLA (protocol number: 2008–084). 3 days prior to implantation, 5 × 105 tested cells were seeded on poly(DL-lactic-co-glycolic acid)/hydroxyapatite (PLGA/HA) scaffolds (diameter: 3-mm; height: 1-mm) and cultured in osteogenic medium for in vitro induction [15]. The detailed procedure of scaffold preparation was described in Supplemental Material and Methods [21]. Non-healing, critical-sized (diameter: 3-mm) calvarial defects were created in the right parietal bone of 8-week old SCID mice under anaesthetization [22]. One defect per animal was created. Cell-free scaffold, scaffold + undifferentiated BJ-fibroblasts, and scaffold + BJ-iPSCs were used as controls. Calvaria were harvested at 8 weeks post-transplantation, fixed in 4% paraformaldehyde (Sigma-Aldrich) for 48 h. High-resolution µCT images were documented (SkyScan1172, SkyScan N.V., Kontich, Belgium) and analyzed by CTAn/CTVol software package provided by the manufacturer [23, 24]. After decalcification with 19% EDTA solution for 21 days, samples were sectioned at 5 µm for histological and immunohistochemical (IHC) evaluation.
2.8. PCR Array
Total RNA from in vitro cultured FReP cells as well as their parental BJ-fibroblasts was extracted using RNeasy® Mini Kit with DNase treatment (Qiagen, Valencia, CA). 0.8 µg RNA was injected into a RT2 First Strand Kit followed by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis in a qBiomaker™ Screening PCR Array or a RT2 Profiler™ PCR Array (Human Osteogenesis; SABiosciences Corp., Valencia, CA), respectively. Three different cDNA templates were tested. qRT-PCR was performed on a 7300 Real-time PCR System (Applied Biosystems, Grand Island, NY). Relative gene expression was evaluated by the manufacturer’s online services (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). Complete gene symbols are listed in Supplementary Table 1.
2.9. Western blot
Cells were harvested with RIPA buffer (Pierce, Rockford, IL) supplemented with Halt™ Protease and Phosphatase Inhibitor Cocktail (Pierce). 15 µg of total protein was loaded onto SDS-PAGE and transferred to polyvinylidene fluoride membrane (Immobilon®-PSQ; Millipore, Billerica, MA). All antibodies used for Western blot are listed in Supplementary Table 2. ECL Western Blotting Substrate (Pierce) was used for development.
2.10. Histological and Immunological staining
Hematoxylin and eosin (H&E) and Masson’s trichrome staining was used to detect global morphology. Alizarin Red staining and Von Kossa staining [23, 24] as well as immunological staining with antibodies against osteogenic markers were used for osteogenic differentiation assessment in vitro. Detailed information about the antibodies used for immunological staining is also provided in Supplementary Table 2. For counter staining, phalloidin (Life Technology) was used for F-actin staining, while 4’,6-diamidino-2-phenylindole (DAPI; Life Technology) was used for nuclear staining.
2.11. Statistical analysis
Statistical analysis was conducted as per consultation with the UCLA Statistical Biomathematical Consulting Clinic. Data were presented as mean ± SD. Data analysis was achieved by OriginPro 8 (Origin Lab Corp., Northampton, MA). P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Purify and increase the reprogramming rate of FReP cells
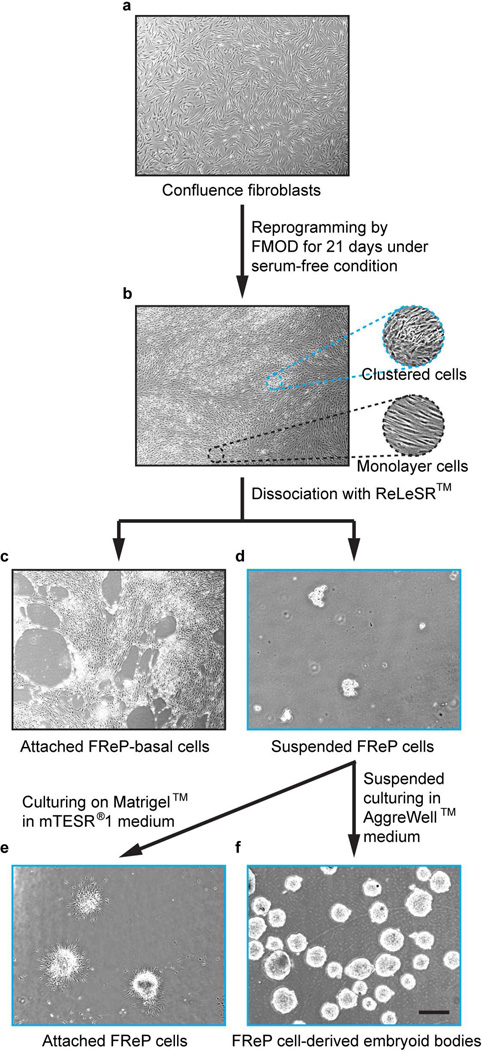
After treatment with FMOD under a serum-free condition for 21 days, a portion of the homogenous spindle-shaped fibroblasts (Fig. 1a) converted to dome-shaped cells and clustered to form multilayer retractile colonies, while the other cells surrounding the colonies maintained the spindle shape and remained in monolayer (Fig. 1b). Using a newly developed, animal component-free and enzyme-free reagent ReLeSR™, which was developed to passage human pluripotent stem cells without manual selection or scraping [18, 19], these two subsets of cells were easily dissociated. Following the incubation with ReLeSR™, monolayer cells (namely FReP-basal cells) remained attached to the culture plate (Fig. 1c), while the reprogrammed FReP cell colonies were lifted off of the culture plate (Fig. 1d).
Fig. 1. FMOD reprogramming and FReP cell purification.
(a) Confluent BJ-fibroblasts were treated with FMOD for 21 days under serum-free conditions. (b) The homogenous spindle-shaped fibroblasts divided into two subsets of cells: dome-shaped cells clustered to form multilayer retractile colonies, and spindle-shaped cells remained in monolayer around clustered colonies. After dissociation of ReLeSR™, (c) spindle-shaped cells remained on the culture plates while (d) the clustered dome-shaped cells were lifted off of the culture plate. The dome-shaped cells formed (e) ESC-like colonies in adherent culture or (f) embryoid body in suspension culture. Bar = 500 µm.
These FReP cells formed ESC-like colonies (Fig. 1e) in the highly specialized, feeder-free mTESR®1 medium, which is widely used for human ESC and human iPSC maintenance [20]. In comparison with the manual scraping method, the ratio of ESC-like clone generation was 0.21% (209 ± 5.1 colonies/100,000 fibroblasts), which was almost 7-fold higher than our previously reported FMOD reprogramming efficacy (0.03%; 32 ± 2.6 colonies/100,000 fibroblasts [15]; Paired-sample t-test, N = 6, P < 1.19 × 10−9). Immunostaining demonstrated the expression of core pluripotent transcriptional regulators NANOG, POU5F1, and SOX2in the yielded FReP cell colonies growth on Matrigel™ (Supplementary Fig. 2). Additionally, podocalyxin (PODXL), the surface antigen of human pluripotent cells [25, 26], was also identified on these FReP cell colonies by two different antibodies TRA-1–60 and TRA-1–80, which recognize proteoglycan epitopes on variants of the same protein (Supplementary Fig. 2). In suspension culture, FReP cells harvested by ReLeSR™ reagent formed stable EBs (Fig. 1f) with the staining of NANOG, POU5F1, and SOX2 (Supplementary Fig. 3a). These FReP cell-derived EBs (FReP-EBs) also spontaneously presented the staining of bone morphogenetic protein 4 (BMP4; Supplementary Fig. 3b), which is essential for mesoderm formation [27, 28], and flt-related receptor tyrosine kinase 1 (FLK1, aka. vascular endothelial growth factor receptor 2; Supplementary Fig. 3b), which is a lateral plate mesoderm marker [29]. Interestingly, although the early ectodermal marker NESTIN [30] was found throughout the entire FReP-EBs, neuron specific βIII-tubulin was mainly observed at the surface of these FReP-EBs (Supplementary Fig. 3c). No significant expression of endoderm markers was observed in FReP-EBs (data not shown); however, FReP cells could differentiate into pancreatic lineage cells that were characterized with the expression of pancreatic and duodenal homeobox 1 (PDX1, aka. insulin promoter factor 1; Supplementary Fig. 4), the marker and essential transcription factor of pancreatic differentiation [31]. These phenomena confirmed that ReLeSR™ reagent-lifted FReP cells have the same multiple lineage differentiation potential as FReP cells harvested by the scratching method reported previously [15]. Additionally, FReP-basal cells expressed only moderate NANOG but none of the other tested pluripotent markers (Supplementary Fig. 2) and did not form stable EBs in suspension culture, which suggested that FReP-basal cells are likely a different type of cell to be further studied. Taken together, we successfully purified and significantly increased the reprogramming rate of the FReP cells by using ReLeSR™ reagent.
3.2. Osteogenic differentiation of FReP cells in vitro
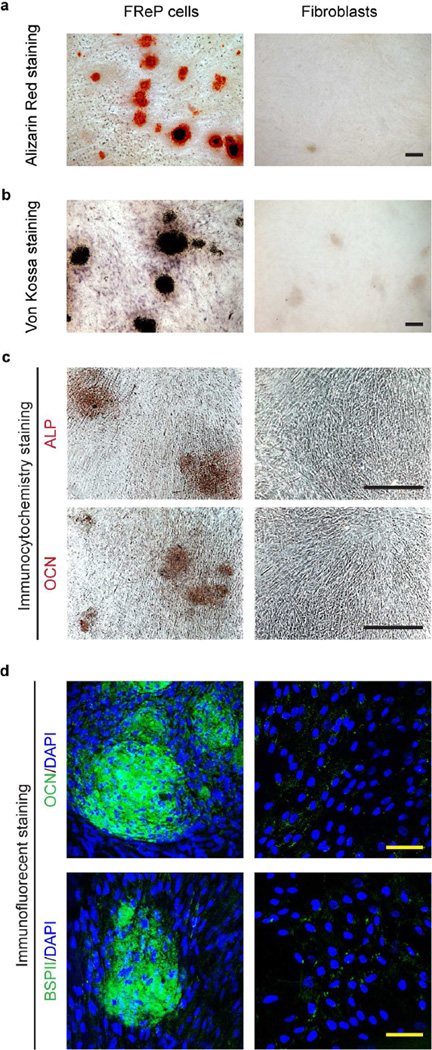
After cultivation in osteogenic differentiation medium in vitro for 4 weeks, both Alizarin Red staining and von Kossa staining demonstrated the mineralization of FReP cells (Fig. 2a–b), which agreed with immunostaining against the broadly accepted major osteogenic markers including alkaline phosphatase (ALP), osteocalcin (OCN), and bone sialoprotein II (BSPII), respectively (Fig. 2c–d). However, under the same situation, no evidence indicated the osteogenic differentiation of their parental BJ-fibroblasts (Fig. 2).
Fig. 2. Osteogenic differentiation of FReP cells after a 4-week cultivation in osteogenic medium in vitro.
(a) Alizarin Red staining, (b) von Kossa staining, (c) immunocytochemistry staining against ALP and OCN, and (d) immunofluorescent staining against OCN and BSPII, respectively. DAPI was used for nuclear staining. Bar = 400 µm (a–c), or 50 µm (d).
3.3. Expression of pluripotent genes in FReP cells during in vitro osteogenic differentiation
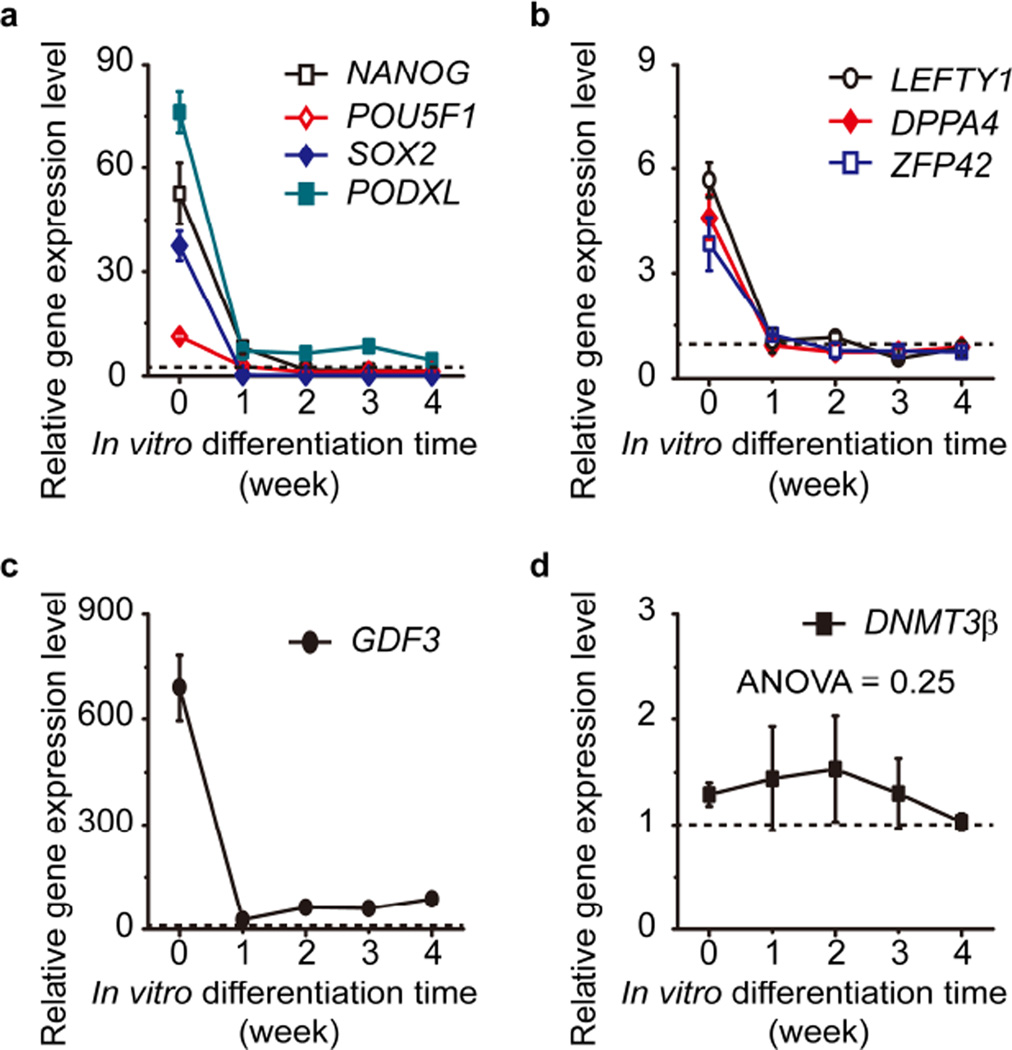
In agreement with the immunostaining results presented previously (Supplementary Fig. 2), qRT-PCR showed the elevated transcription levels of NANOG, POU5F1, SOX, and PODXL in FReP cells in comparison with those of their parental BJ-fibroblasts (Fig. 3a). During the in vitro osteogenic differentiation, these genes were significantly reduced (Fig. 3a). qBiomaker™ Screening PCR Array also revealed that the other subset of pluripotent markers, including left-right determination factor 1 (LEFT1), developmental pluripotency associated 4 (DPPA4), and zinc finger protein 42 (ZFP42) (Fig. 3b), which were significantly increased in undifferentiated FReP cells, had been rapidly downregulated to the same levels of their parental BJ-fibroblasts due to the osteogenic differentiation (Fig. 3a–b). Interestingly, another pluripotency marker growth differentiation factor 3 (GDF3), which was also dramatically induced in FMOD reprogramming, exhibited a specific, biphasic expression profile characterized by a sharp decrease in the first week of osteogenic differentiation followed by a slower elevation stage from then on (Fig. 3c). However, the general GDF3 levels were significantly higher than BJ-fibroblasts during the entire reprogramming and osteogenic differentiation (Fig. 3c). Western blotting results confirmed the expression of these pluripotent markers (Supplementary Fig. 5), which demonstrated the loss of pluripotency of FReP cells during osteogenic differentiation.
Fig. 3. Expression of genes related to pluripotency in FReP cells during osteogenic differentiation in vitro.
(a) NANOG, POU5F1, SOX2, and PODXL; (b) LEFTY1, DPPA4, and ZFP42; (c) GDF3; and (d) DNMT3β, SOX2 expression was analyzed with TaqMan® Gene Expression Assays (Life Technologies) and SsoFast™ Probes Supermix with ROX (Bio-Rad Laboratories)using three different cDNA templates obtained with iScript™ Reverse Transcription Supermix forqRT-PCR (Bio-Rad Laboratories). Expression of other genes was analyzed by qBiomaker™ Screening PCR Array (Qiagen) using three different cDNA templates. Concomitant GAPDH was used as a housekeeping standard. Data were normalized to un-reprogrammed BJ-fibroblasts (black dotted line). One-way ANOVA and Two-sample t-test were used to compare the data statistically (N = 3).
Surprisingly, DNA (cytosine-5-)-methyltransferase 3β (DNMT3β), which was highly expressed in ESCs, exhibited consistently relatively low transcription levels in FReP cells regardless of reprogramming and osteogenic differentiation (Fig. 3d). This result supported the hypothesis that DNMT3β is dispensable for pluripotent/multipotent cell reprogramming and maintenance [32–34]. Moreover, since hypermethylation of genomic DNA by DNMT3α and DNMT3β is critical for ECSs to form teratomas in vivo [35], the consistently low level of DNMT3β during FMOD reprogramming may have contributed to the low tumorigenic potential of FReP cells in addition to low c-MYC and high p15 and p21 levels reported previously [15].
3.4. Osteogenic gene profile of FReP cells during in vitro osteogenic differentiation
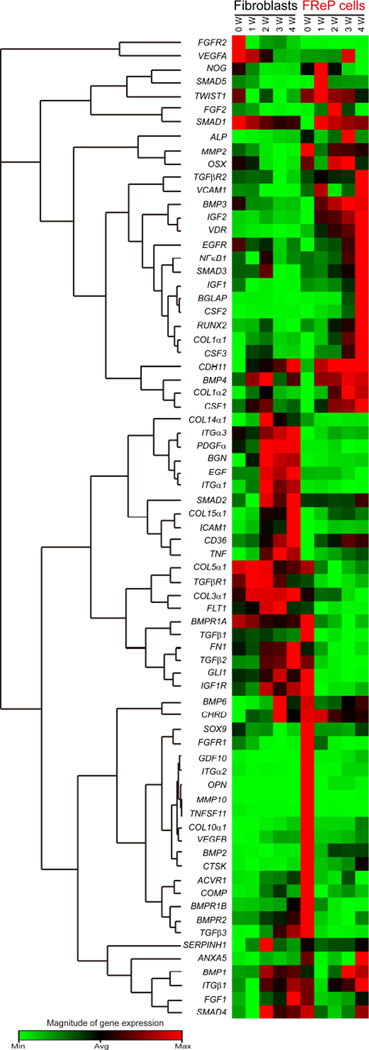
PCR arrays are the most reliable tools for analyzing the expression of a focused panel of genes with reasonable costs. Using a commercially available Human Osteogenesis RT2 Profiler PCR Array, we evaluated the expression of 84 genes related to osteogenic differentiation in FReP cells with their parental BJ-fibroblasts as control (Fig. 4 and Supplementary Table 3). Expression of certain specific sets of genes was described below:
Fig. 4. Expression of genes related to the development of the skeletal system as well as bone mineral metabolism during osteogenic differentiation in vitro.
Gene expression was analyzed by RT2 Profiler™ PCR Array (Human Osteogenesis, Qiagen). Concomitant GAPDH was used as a housekeeping standard. Genes with extremely low expression levels (average threshold cycle is either undetermined or greater than the cut-off value of 35 cycles), including αHSG, BMP5, BMP7, CALCR, COL2α1, DLX5, IHH, ITGαM, MMP8, MMP9, and PHEX (Supplementary Table 3), were omitted from the heat map cluster. (N=3)
Osteoblast commitment and differentiation are regulated by diverse growth factors [36]. Among them, transforming growth factor (TGF)βs and their family members, such as BMPs, have been implicated in both maintenance and differentiation of pluripotent cells [37]. Thus, we first assessed the expression of TGFβ family members during FReP cell osteogenic differentiation in vitro. In comparison with BJ-fibroblasts, undifferentiated FReP cells presented significantly higher levels of all three TGFβ isoforms (Supplementary Fig. 6a–c). Expression of TGFβs sharply decreased in the FReP cells during the first week of osteogenic differentiation, and then maintained low levels throughout the entire in vitro osteogenic differentiation (Supplementary Fig. 6a–c). On the contrary, BJ-fibroblasts had consistent TGFβ1 levels during the entire four-week cultivation, while the expression levels of TGFβ2 and TGFβ3 continually increased throughout weeks 2–4 (Supplementary Fig. 6a–c). Meanwhile, FReP cells had significantly higher BMP2 levels than their parental BJ-fibroblasts during the entire four-week osteogenic differentiation (Supplementary Fig. 6d). Interestingly, BMP2 presented a specific, three-phasic expression pattern in FReP cells characterized by significantly reduced levels at week 1, followed by a moderate increase at week 2, and then maintained the same level during the last two weeks (Supplementary Fig. 6d). Undifferentiated FReP cells have lower levels of BMP4 than fibroblasts, but BMP4 expression was significantly upregulated in FReP cells in week 1 of osteogenic differentiation and kept at that level thereafter (Supplementary Fig. 6e). In addition to the TGFβ family, the insulin-like growth factor (IGF) family also stimulates osteoblast function and bone matrix deposition [38]. In this study, we found that expression of IGF1 was reduced in BJ-fibroblasts during week 1 of cultivation and kept at an extremely low level afterwards (Supplementary Fig. 6f). In FReP cells, IGF1 maintained consistent levels throughout weeks 1–3 of osteogenic differentiation followed by a significant increase in the last week (Supplementary Fig. 6f). On the other hand, BJ-fibroblasts had a stable IGF2 expression, while FReP cells had a continually elevated expression of IGF2 during the entire osteogenic differentiation (Supplementary Fig. 6g). Fibroblast growth factor (FGF)2 (aka. basic fibroblast growth factor) also plays an essential role in promoting the conversion of uncommitted pluripotent/multipotent cells to osteochondroprogenitors and the subsequent osteogenic differentiation via multiple pathways [28, 39–45]. Transcription of FGF2 was markedly reduced in week 2 when BJ-fibroblasts were cultured in the osteogenic medium (Supplementary Fig. 6h). On the contrary, the transcription of FGF2 significantly increased in FReP cells during weeks 1 and 2 of osteogenic differentiation followed by an obvious drop in weeks 3 and 4 (Supplementary Fig. 6h).
At the transcriptional factor level, FMOD reprogramming significantly downregulated the transcription of BMP-responsive transcriptional factor SMAD1 (Supplementary Fig. 7a). However, the expression of SMAD1 was recovered in FReP cells in week 1 of osteogenic differentiation and kept at higher levels than those of BJ-fibroblasts afterward (Supplementary Fig. 7a). Although expression of SMAD5, another essential BMP-responsive transcriptional factor, was not influenced by the FMOD reprogramming, FReP cells exhibited higher SMAD5 levels than parental BJ-fibroblasts when cultured in osteogenic medium (Supplementary Fig. 7b). The transcription of TWIST1 and SOX9, which are required for osteochondroprogenitor lineage specification [46–48], was significantly induced in FReP cells in week 1 of osteogenic differentiation and dropped back to the basal levels in week 2, while expression of TWIST1 and SOX9 was kept at low levels in BJ-fibroblasts during the entire four-week in vitro cultivation (Supplementary Fig. 7c,d). As a master transcriptional activator of osteoblast differentiation [46, 49], RUNX2 (originally called Cbfa1) was stimulated in FReP cells throughout the four-week osteogenic period, especially weeks 3–4 (Supplementary Fig. 7e). Interestingly, in FReP cells, the transcription factor osterix (OSX), which is regulated by RUNX2 and is required for mature bone formation [50], was down-regulated in week 1 of osteogenic differentiation, followed by an increase in weeks 2 and 3 before dropping back to the basal level in week 4 (Supplementary Fig. 7e). Meanwhile, the transcription of OSX decreased in BJ-fibroblasts during the entire four-week in vitro cultivation (Supplementary Fig. 7f). Vitamin D receptor (VDR), which is a member of the nuclear receptor superfamily of transcription factors that is highly expressed during stem cell osteogenic differentiation [51], was also significantly induced in FReP cells throughout the in vitro osteogenic differentiation period (Supplementary Fig. 7g). At the same time, the transcription of VDR was continually decreased in BJ-fibroblasts (Supplementary Fig. 7g).
With regard to the extracellular matrix (ECM), type I collagen comprises approximately 80% of the total proteins present in bone [52], and its expression was constantly upregulated in FReP cells instead of BJ-fibroblasts during the in vitro osteogenic differentiation (Supplementary Fig. 8a,b). Interestingly, transcription of osteopontin (OPN; which is important for biomineralization [53] and anchoring osteoclasts to the mineral matrix of bones [54]) and ALP, a tissue-nonspecific isozyme (which is presumed to be involved in the calcification of bone matrix [55]; encoded by gene ALPL), in FReP cells was similar to that of OSX: decreased in week 1, up-regulated in weeks 2 and 3, and down-regulated again in week 4; while, in BJ-fibroblasts, both OPN and ALPL maintained low expression levels (Supplementary Fig. 8c,d). On the other hand, transcription of OCN (aka. bone γ-carboxyglutamic acid-containing protein; which is secreted solely by osteoblasts [56] and encoded by the gene BGLAP), was stimulated in FReP cells but not BJ-fibroblasts in weeks 3 and 4 of in vitro osteogenic differentiation (Supplementary Fig. 8e). Taken together, the gene profiling data agreed with our immunological staining data presented above (Fig. 2) and confirmed the osteogenic differentiation of FReP cells in vitro.
3.4. Implantation of FReP cells in a critical-sized calvarial defect model
Our previous studies have shown that implantation of FReP cells into a pocket in the gluteofemoral muscle of SCID mice with osteoinductive demineralized bone matrix (DBM) resulted in osteogenic differentiation in vivo [15]. As we noticed that DBM is an osteoinductive scaffold, which contains osteogenic growth factors, such as BMP2, and multiple undefined growth factors that holding the potential to induce osteogenesis as well as undesired side toxic effects [57]. In order to test the feasibility of FReP cells as an alternative cell source for bone regeneration in a more challenging clinically relevant traumatic scenario, FReP cells were seeded on osteoconductive PLGA/HA scaffolds and implanted into a critical-sized SCID mouse calvarial defect. PLGA-HA scaffold was chosen in the current study instead of DBM scaffold to eliminate the osteoinductive stimulation of the growth factors on the DBM scaffold.
3.4.1. Radiography
As proof of concept in the critical-sized calvarial defect model, cell-free scaffold alone did not induce obvious bone regeneration at 8 weeks post-implantation (Fig. 5a). As described above, all tested cell types (BJ-fibroblasts, BJ-iPSCs, and FReP cells) were seeded on the PLGA/HA scaffold and underwent a 3-day in vitro osteogenic initiation before implantation. By using MTT assay as previously described [58], we found no significant difference on adhesion cell numbers between the three groups prior to implantation (One-way ANOVA test, N = 6, P = 0.71).
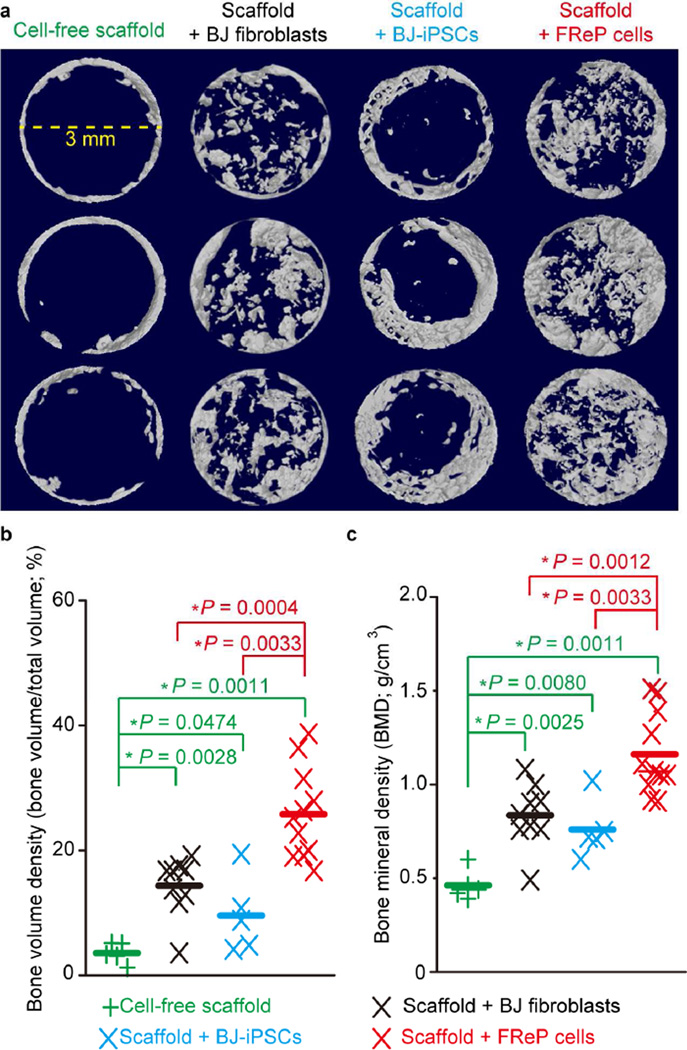
Fig. 5. Radiographic analysis of bone regeneration in critical-sized SCID mouse calvarial defects at week 8 post-implantation.
(a) µCT image of bone regeneration in critical-sized mouse calvarial defects implanted with cell-free scaffold (N = 5), scaffold + undifferentiated BJ-fibroblasts (N = 9), scaffold + BJ-iPSCs (N = 5), and scaffold + FReP cells (N = 11). 5 × 105 cells were seeded on PLGA/HA scaffold and cultured in osteogenic medium 3 days prior to implantation. Images were documented at a resolution of 20.0 µm. (b) Bone volume density and (c) bone mineral density quantification revealed that implantation of FReP cells resulted in significantly more bone formation than other groups in critical-sized SCID mouse calvarial defects at week 8 post-transplantation. *, significant difference revealed by Mann-Whitney test; green stars indicate the significance from the cell-free scaffold; red stars indicate the significance in comparison to scaffold + FReP cells.
Although complete defect healing was not observed in any of the tested groups at 8 weeks post-implantation, significantly more bone formation was observed in the group implanted with FReP cells than the groups implanted with BJ-fibroblasts or BJ-iPSCs (Fig. 5a), which was further confirmed by the quantification of bone volume density (bone volume/total volume, BV/TV; Fig. 5b) and bone mineral density (BMD; Fig. 5c). It is worth noting that, while newly formed bone tissue was detected throughout the entire defect in both the BJ-fibroblast group and the FReP cell group, bone formation in the BJ-iPSC group was limited at the edge of the defects (Fig. 5a).
3.4.2 Histological and IHC analyses
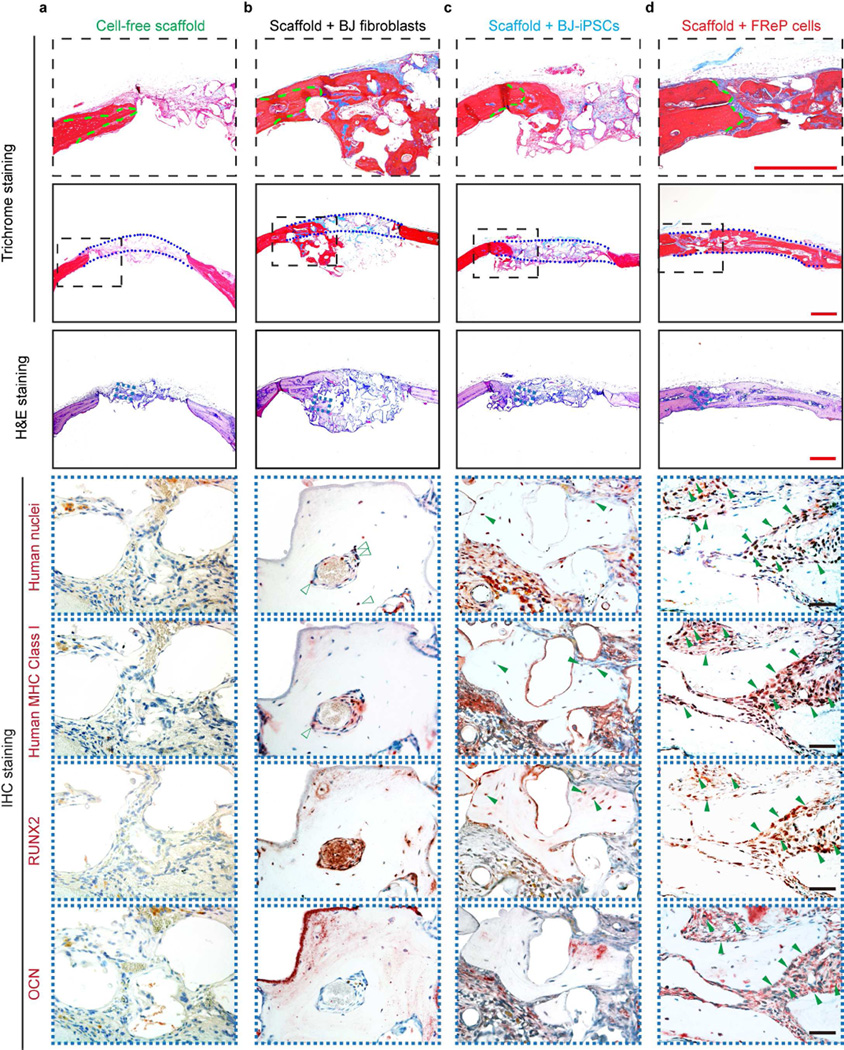
Consistent with radiographic analysis, there was minimal bone regeneration in the group implanted with cell-free scaffold alone (Fig. 6a). In the BJ-fibroblast group, bone formation was not restricted in the defect area, but also escaped the defect and extended underneath with obvious ‘cyst-like bone voids’ in the newly generated bone tissue and the defect (Fig. 6b), which contributed to the relative low BMD value (Fig. 5c). In agreement with the radiographic image, the new bone tissue was predominantly observed at the edge of the defects in the BJ-iPSC group (Fig. 6c). However, a mineralized bony bridge connecting the two defect ends without ectopic bone formation was clearly identified by H&E and Masson’s trichrome staining in the FReP cell group (Fig. 6d).
Fig. 6. Engraftment, persistence, and osteogenesis of FReP cells in critical-sized SCID mouse calvarial defects at week 8 post-implantation.
(a) H&E and Masson’s trichrome staining confirmed that only minimal bone regeneration occurred in the group implanted with cell-free scaffold alone, while (b) implantation of BJ-fibroblasts resulted in bone formation underneath the calvarial defect with obvious ‘cyst-like bone voids’ in the newly generated bone tissue. (c) The newly formed bone tissue was predominantly observed at the edge of the defects in BJ-iPSC group. (d) On the contrary, implantation with FReP cells led to a mineralized bony bridge connecting the two defect ends without ectopic bone formation. In Masson Trichrome staining, the matured bone is stained in red, and osteoid is stained in blue. Green dotted lines outlined the initial edges of the calvarial defect, while blue dotted lines outlined the implantation area, respectively. Furthermore, immunostaining of human nuclei and MHC Class I as well as osteogenic markers, RUNX2 and OCN, revealed BJ-iPSCs and FReP cells underwent osteogenic differentiation (solid green arrows) in active osteogenic regions of the defects, while BJ fibroblasts (open green arrows) were only detected in the fibrosis area instead of newly formed bone tissues. Bar = 500 µm (red) or 50 µm (black).
Meanwhile, human cells (BJ-fibroblasts, BJ-iPSCs, and FReP cells) survived in newly generated bone tissue of SCID mouse calvarial defects at 8 weeks post-implantation and were identified by antibodies against human nuclei and human major histocompatibility complex (MHC) Class I (Fig. 6). However, immunohistochemical staining revealed that there is no significant overlap between the human cell marker staining and osteogenic differentiation marker (RUNX2 and OCN) staining in the BJ-fibroblast group (Fig. 6b), which indicated implanted fibroblasts may only function as a paracrine signal provider to support calvarial defect healing instead of engrafting into the newly formed bone tissue. On the contrary, the spatial co-localization of human cell markers with osteogenic markers was detected in the osteogenic regions of the defects in both BJ-iPSC group and FReP cell group (Fig. 6c,d), which confirmed the engraftment and differentiation of BJ-iPSCs and FReP cells in vivo. Considering the significantly more bone formation with higher density in the FReP cell group than that of the BJ-iPSC group, FReP cells presented a significant advantage in bone regeneration efficacy compared with parental BJ-fibroblasts and BJ-iPSCs.
4. Discussion
Pluripotent or multipotent cell-based therapeutics are vital for skeletal reconstruction in non-healing critical-sized defects [4–6, 59, 60]. A principle challenge is to produce enough regenerative cells through a simple, consistent approach that bypasses ethical concerns and allogeneic immune rejection and avoids genomic alteration for in vivo bone formation. In addition, from a FDA point of view, all cellular and gene therapy (CGT) products must fulfill the prescribed requirements of purity, potency, and safety. ESCs do not meet these requirements since ESCs have the potential risk of rejection owing to their allogeneic nature [61] and tumorigenesis [7]. Although MSCs have been proposed as potential cell sources for bone regeneration [59, 60], the low stem cell harvest rate and highly variable multipotency caused by donor variability (particularly in the aged or osteoporotic population, whose MSC number and differentiation capability are considerably reduced) significantly diminish the efficacy of MSC-based therapies [62–64]. In addition, the traditional avenues of MSC derivation, which include bone marrow aspiration, liposuction, and less commonly muscle biopsy, are all more invasive and entail potentially more pain and medical or surgical risks, such as bleeding and anesthesia, than a simple skin biopsy [4–6]. On the other hand, iPSCs can be derived directly from dermal fibroblasts, which are easily obtained and expanded from skin biopsies [17, 65]. Moreover, there is already a Food and Drug Administration (FDA) approved product for autologous dermal fibroblast expansion and injection (www.fibrocellscience.com), which confirmed the safety of autologous dermal fibroblast application [66]. However, because the introduction of transcriptional factors essential for embryonic development (such as Yamanaka factors or Thomson factors) into the genome of target somatic cells is essential for classic iPSC generation, and such a process may involve unwanted gene activation and interruption from viral integration, iPSCs are likely to carry an even higher risk of tumorigenesis than ESCs [8–12]. The non-integrative iPSC generation techniques, such as those using adenoviruses, DNA, and oocytes or ESCs, are complicated by a plethora of disadvantages including cell penetration, cytosolic delivery, sensitivity to reagents, intensive labor, and contamination with non-human molecules, and do not eliminate the risk of tumorigenesis, which remains a significant barrier to safe clinical application of iPSCs [10–12, 67–71]. Thus, current cell-based strategies do not safely and adequately satisfy the requirements of human skeletal muscle and bone tissue engineering.
Previously, we have demonstrated that, under serum-free conditions, continuous treatment with FMOD is sufficient to reprogram human dermal fibroblasts into quiescent stem cell-like FReP cells with the capacity to differentiate into multiple lineage derivatives [15]. More importantly, since FMOD reprogramming does not involve either genomic alteration or oncogene participation, the FReP cells do not form teratomas in vivo [15], which makes FReP cells a much safer cell source than iPSCs for tissue regeneration. In the current study, by using an enzyme-free hESC and hiPSC selection and passaging reagent, ReLeSR™, we further purified FReP cells by eliminating the non-fully reprogrammed FReP-basal cells and observed a significant 7-fold increasein FReP cell colony formation. Moreover, we also demonstrated that FReP cells could differentiate into osteoblasts in vitro and successfully formed bone tissue in vivo without the induction of tumorigenesis in both intramuscular (muscle pouch [15]) and bone (calvarial) defect SCID mouse models. It is worth noting that the FReP cell-based in vivo bone formation is not reliant on exogenous osteogenic growth factors, such as BMP2, which could be delivered directly or released from the osteoinductive DBM scaffold [72]. Moreover, an inadequate dose of BMP2 could induce adverse clinical effects such as life-threatening inflammation and ectopic bone formation with neurologic impairment [73, 74]. Additionally, osteoinductive scaffolds with undefined composition, such as DBM, could also increase the risk for undesired side-effects such as host immune response and disease transmission [57]. By avoiding the exogenous application of BMP2 and DBM, FReP cell-based therapies will provide a potentially safe alternative route to treat delicate bone defects, especially for treatment of calvarial defects in close proximity to the brain.
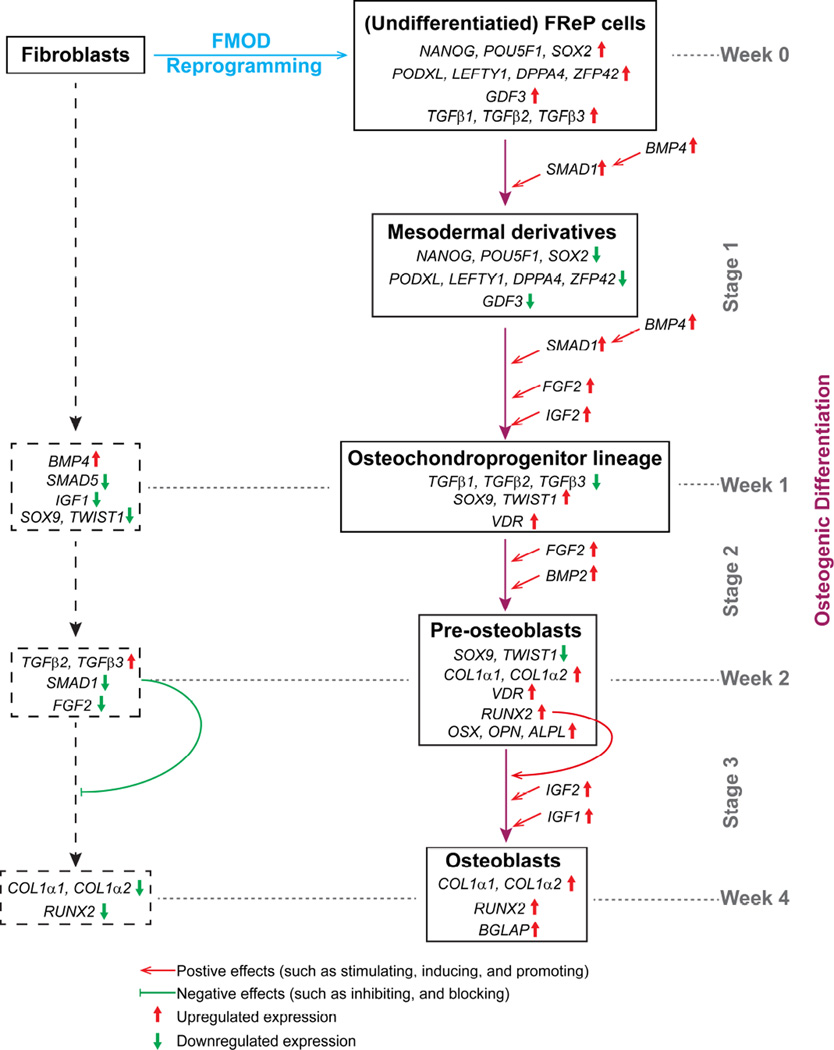
Moreover, in the current study, we revealed a rough three-stage ‘molecular blueprint’ of FReP cell osteogenic differentiation by gene profiling (Fig. 7):
Stage 1 (week 1): Due to the stimulation of osteogenic media, the pluripotent markers and TGFβs, which are important for pluripotency maintenance[37], were also significantly decreased in FReP cells (Figs. 3, and Supplementary Figs. 5, 6a–c). On the other hand, due to the elevation of autocrine BMP4 (Supplementary Fig. 6e), which can induce mesodermal differentiation [27, 75], FReP cells underwent mesodermal differentiation. Then, under the combo stimulation of BMP4, FGF2, and IGF2 (Supplementary Figs. 6e–g), like MSCs [36, 41, 75–78], FReP cells further converted to osteochondroprogenitors with increased TWIST1, SOX9, and VDR levels (Supplementary Figs. 7c,d,g). Surprisingly, BMP2 was significantly down-regulated in FReP cells in week 1 of osteogenic differentiation (Supplementary Figs. 6d), accompanied by its functional competitor GDF3 (Fig. 3c and Supplementary Figs. 5c) [79, 80]. Previous studies suggested that the balance between BMP2 and GDF3 dominates the fate of pluripotent cells [81], thus the elevated BMP2/GDF3 ratio (Supplementary Fig. 9) may also contribute to the osteochondroprogenitor commitment of FReP cells. On the other hand, TWIST1 and SOX9 were down-regulated in BJ-fibroblasts in the same situation (Supplementary Figs. 7c–d), indicating that BJ-fibroblasts did not convert to osteochondroprogenic lineage cells.
Stage 2 (week 2): In this stage, under the influence of endogenous BMP2 and FGF2[45], FReP cells differentiated into pre-osteoblasts with increased osteogenic markers, such as RUNX2, OSX, OPN, ALPL, and type I collagen (Supplementary Figs. 7e–f and Supplementary Figs. 8a–d). However, down-regulation of SOX9 and TWIST1 in FReP cells was observed (Supplementary Figs. 7c–d) as the requirement for osteoblast differentiation and mineralization [47, 82].
Stage 3 (week 3–4): In this stage, IGF1 and IGF2 [36, 76] dominated the FReP cell-derived osteoblast maturation in this stage (Supplementary Figs. 6f–g), and resulted in osteoblast maturation and later mineralization, which was characterized by further type I collagen accumulation (Supplementary Figs. 8a–b) and rapidly induced OCN expression (Supplementary Fig. 8e).
Fig. 7.
‘Molecular blueprint’ of FReP cell osteogenic differentiation.
In summary, we have generated novel multipotent FReP cells by exposing human dermal fibroblasts to FMOD under serum-free scenarios without genomic alteration or oncogene participation. In this study, we further increased the purity and reprogramming rate of FReP cells by using an enzyme-free selection and passaging reagent. By profiling the gene expression during FReP cell osteogenesis, we uncovered the ‘molecular blueprint’ of FReP cell osteogenic differentiation. More importantly, we demonstrated the robust osteogenic capacity of FReP cells in a clinically relevant animal model making them a promising candidate for bone tissue regeneration. No doubt, considering the short history for FMOD reprogramming investigation, many more studies are warranted to enrich our knowledge about FReP cells to the levels that we understand currently available stem cells, including further clarifying the FMOD reprogramming mechanism, and revealing the potential immune-modulation and paracrine function of FReP cells. Additionally, more extensive investigation will be required to translate FReP cell investigation from bench characterization to clinical application, including, but not limited to, optimizing the cell seeding density and culture procedure [83–86], minimizing the xenogeneic exposure for in vitro osteogenic initiation, enhancing the properties of the supporting osteoconductive scaffold(s), improving the interaction between FReP cells and scaffolds [85, 86], and large animal efficacy and safety tests.
5. Conclusion
We have pioneered the induction of multipotency in somatic cells by using only a single proteoglycan, FMOD, without gene transduction. In the current study, we further purified and significantly increased the reprogramming rate of FReP cells by eliminating the non-fully reprogrammed FReP-basal cells with a newly developed, animal component-free and enzyme-free hESC and hiPSC selection and passaging reagent ReLeSR™. Moreover, by demonstrating the potency, safety, and ‘molecular blueprint’ of FReP cell-based bone regeneration, we are confident that FReP cells present a high potential for CGT products for bone regeneration, which supported the hypothesis that FMOD reprogramming has the potential to shift the paradigm of reprogramming autologous cells for tissue reconstruction into a much safer protein-based process.
Supplementary Material
Acknowledgments
This study was supported by the UCLA and Orthopaedic Hospital Department of Orthopaedic Surgery and the Orthopaedic Hospital Research Center, the Eli & Edy Broad Center of Regenerative Medicine and Stem Cell Research at UCLA Innovation Award, the National Center for Advancing Translational Sciences UCLA CTSI Grant (UL1TR000124), the Faculty seed grant from UCLA School of Dentistry, the Plastic Surgery Foundation® (2013 National Endowment for Plastic Surgery269698), NIH-NIDCR (R44DE024692), NIH-NIAMS (R44AR064126) and the International S&T Cooperation Program of China (Grant No. 2013DFB30360). Confocal laser scanning microscopy was performed at the Center for NanoScience Institute Advanced Light Microscopy/Spectroscopy Shared Resource Facility at UCLA, which was supported by funding from the NIH-NCRR shared resources grant (CJX1-443835-WS-29646) and the NSF Major Research Instrumentation grant (CHE-0722519).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
Drs. Kang Ting, Zhong Zheng, and Chia Soo are inventors of fibromodulin-related patents filed from UCLA. Drs. Kang Ting, Zhong Zheng, and Chia Soo are founders of Scarless Laboratories Inc. which sublicenses fibromodulin-related patents from the UC Regents, who also hold equity in the company. Drs. Zhong Zheng and Chia Soo are also officers of Scarless Laboratories, Inc. K.T., C.S., and Z.Z. = Kang Ting, Chia Soo, Zhong Zheng
References
- 1.Petite H, Viateau V, Bensaid W, Meunier A, de Pollak C, Bourguignon M, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–963. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 2.Wieser K, Zingg P, Dora C. Trochanteric osteotomy in primary and revision total hip arthroplasty: risk factors for non-union. Archives of orthopaedic and trauma surgery. 2012;132:711–717. doi: 10.1007/s00402-011-1457-4. [DOI] [PubMed] [Google Scholar]
- 3.Park MS, Kim SS, Cho SW, Choi CY, Kim BS. Enhancement of the osteogenic efficacy of osteoblast transplantation by the sustained delivery of basic fibroblast growth factor. J Biomed Mater Res B. 2006;79B:353–359. doi: 10.1002/jbm.b.30549. [DOI] [PubMed] [Google Scholar]
- 4.Hjortholm N, Jaddini E, Halaburda K, Snarski E. Strategies of pain reduction during the bone marrow biopsy. Annals of hematology. 2013;92:145–149. doi: 10.1007/s00277-012-1641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YH, Cha SM, Naidu S, Hwang WJ. Analysis of Postoperative Complications for Superficial Liposuction: A Review of 2398 Cases. Plastic And Reconstructive surgery. 2011;127:863–871. doi: 10.1097/PRS.0b013e318200afbf. [DOI] [PubMed] [Google Scholar]
- 6.Lu SH, Wei CF, Yang AH, Chancellor MB, Wang LS, Chen KK. Isolation and Characterization of Human Muscle-derived Cells. Urology. 2009;74:440–445. doi: 10.1016/j.urology.2009.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 9.Walia B, Satija N, Tripathi RP, Gangenahalli GU. Induced Pluripotent Stem Cells: Fundamentals and Applications of the Reprogramming Process and its Ramifications on Regenerative Medicine. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9279-x. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Okita K, Yamanaka S. Induced pluripotent stem cells: opportunities and challenges. Philos T R Soc B. 2011;366:2198–2207. doi: 10.1098/rstb.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Iozzo RV, Goldoni S, Berendsen AD, Young MF. In: Small Leucine-Rich Proteoglycans: The Extracellular Matrix: an Overview. Mecham RP, editor. Springer Berlin Heidelberg; 2011. pp. 197–231. [Google Scholar]
- 14.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Z, Jian J, Zhang X, Zara JN, Yin W, Chiang M, et al. Reprogramming of human fibroblasts into multipotent cells with a single ECM proteoglycan, fibromodulin. Biomaterials. 2012;33:5821–5831. doi: 10.1016/j.biomaterials.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Z, Nguyen C, Zhang X, Khorasani H, Wang JZ, Zara JN, et al. Delayed wound closure in fibromodulin-deficient mice is associated with increased TGF-beta3 signaling. J Invest Dermatol. 2011;131:769–778. doi: 10.1038/jid.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thuc L, Kim KP, Fan GP, Faull KF. A sensitive mass spectrometry method for simultaneous quantification of DNA methylation and hydroxymethylation levels in biological samples. Analytical biochemistry. 2011;412:203–209. doi: 10.1016/j.ab.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A Technical Video Tip for the Successful Tapping Technique When Using ReLeSR™. STEMCELL Technologies. 2014 Available from: http://www.stemcell.com/en/Technical-Resources/95a2e/A-Technical-Video-Tip-for-the-Successful-Tapping-Technique-When-Using-ReLeSR.aspx.
- 19.ISSCR 2014 Innovation Showcase - Scalable Enzyme-Free Protocols for the Isolation and Maintenance of Human Induced Pluripotent Stem Cells (hiPSCs) Without Mechanical Colony Scraping. STEMCELL Technologies. 2014 Available from: http://www.stemcell.com/en/Forms/Multimedia/Archived-Webinar-Scalable-Enzyme-Free-Protocols-for-the-Isolation-and-Maintenance-of-hiPSCs.aspx?id=
- 20.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou YF, Dunn JC, Wu BM. In vitro response of MC3T3-E1 pre-osteoblasts within three-dimensional apatite-coated PLGA scaffolds. Journal of biomedical materials research Part B, Applied biomaterials. 2005;75:81–90. doi: 10.1002/jbm.b.30261. [DOI] [PubMed] [Google Scholar]
- 22.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nature Biotechnology. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Peault B, Chen W, Li W, Corselli M, James AW, et al. The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng Part A. 2011;17:2497–2509. doi: 10.1089/ten.tea.2010.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Kuroda S, Carpenter D, Nishimura I, Soo C, Moats R, et al. Craniosynostosis in transgenic mice overexpressing Nell-1. J Clin Invest. 2002;110:861–870. doi: 10.1172/JCI15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Draper JS, Pigott C, Thomson JA, Andrews PW. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. Journal of anatomy. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schopperle WM, DeWolf WC. The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells. 2007;25:723–730. doi: 10.1634/stemcells.2005-0597. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 28.Lee TJ, Jang J, Kang S, Jin M, Shin H, Kim DW, et al. Enhancement of osteogenic and chondrogenic differentiation of human embryonic stem cells by mesodermal lineage induction with BMP-4 and FGF2 treatment. Biochem Biophys Res Commun. 2013;430:793–797. doi: 10.1016/j.bbrc.2012.11.067. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1992 Jan;114(1):253–259. doi: 10.1242/dev.118.2.489. 118;489–98. [DOI] [PubMed] [Google Scholar]
- 30.Sidhu KS, Tuch BE. Derivation of three clones from human embryonic stem cell lines by FACS sorting and their characterization. Stem Cells Dev. 2006;15:61–69. doi: 10.1089/scd.2006.15.61. [DOI] [PubMed] [Google Scholar]
- 31.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 32.Pawlak M, Jaenisch R. De novo DNA methylation by Dnmt3a and Dnmt3b is dispensable for nuclear reprogramming of somatic cells to a pluripotent state. Genes & Development. 2011;25:1035–1040. doi: 10.1101/gad.2039011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wongtrakoongate P, Li JL, Andrews PW. DNMT3B inhibits the re-expression of genes associated with induced pluripotency. Experimental Cell Research. 2014;321:231–239. doi: 10.1016/j.yexcr.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Liao J, Karnik R, Gu HC, Ziller MJ, Clement K, Tsankov AM, et al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nature Genetics. 2015;47:469–U464. doi: 10.1038/ng.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen TP, Ueda Y, Dodge JE, Wang ZJ, Li E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Molecular And Cellular Biology. 2003;23:5594–5605. doi: 10.1128/MCB.23.16.5594-5605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E, et al. Bone regeneration and stem cells. Journal of cellular and molecular medicine. 2011;15:718–746. doi: 10.1111/j.1582-4934.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 38.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocrine reviews. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochiai-Shino H, Kato H, Sawada T, Onodera S, Saito A, Takato T, et al. A novel strategy for enrichment and isolation of osteoprogenitor cells from induced pluripotent stem cells based on surface marker combination. PLoS One. 2014;9:e99534. doi: 10.1371/journal.pone.0099534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 41.Ito T, Sawada R, Fujiwara Y, Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology. 2008;56:1–7. doi: 10.1007/s10616-007-9092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagmann S, Moradi B, Frank S, Dreher T, Kammerer PW, Richter W, et al. FGF-2 addition during expansion of human bone marrow-derived stromal cells alters MSC surface marker distribution and chondrogenic differentiation potential. Cell Prolif. 2013;46:396–407. doi: 10.1111/cpr.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spector JA, Mathy JA, Warren SM, Nacamuli RP, Song HM, Lenton K, et al. FGF-2 acts through an ERK1/2 intracellular pathway to affect osteoblast differentiation. Plast Reconstr Surg. 1995 Nov;96(6):1251–1259. doi: 10.1097/01.prs.0000153035.73507.7b. discussion 1260–1. 115;838–52. [DOI] [PubMed] [Google Scholar]
- 44.Fei YR, Xiao LP, Doetschman T, Coffin DJ, Hurley MM. Fibroblast Growth Factor 2 Stimulation of Osteoblast Differentiation and Bone Formation Is Mediated by Modulation of the Wnt Signaling Pathway. Journal Of Biological Chemistry. 2011;286:40575–40583. doi: 10.1074/jbc.M111.274910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agas D, Sabbieti MG, Marchetti L, Xiao L, Hurley MM. FGF-2 enhances Runx-2/Smads nuclear localization in BMP-2 canonical signaling in osteoblasts. J Cell Physiol. 2013;228:2149–2158. doi: 10.1002/jcp.24382. [DOI] [PubMed] [Google Scholar]
- 46.Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genom Hum G. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- 47.Bialek P, Kern B, Yang XL, Schrock M, Sosic D, Hong N, et al. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–435. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- 48.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. J Bone Miner Res. 2002;17:S142–S142. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 50.Nakashima K, Zhou X, Kunkel G, Zhang ZP, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 51.Olivares-Navarrete R, Sutha K, Hyzy SL, Hutton DL, Schwartz Z, McDevitt T, et al. Osteogenic Differentiation of Stem Cells Alters Vitamin D Receptor Expression. Stem Cells Dev. 2012;21:1726–1735. doi: 10.1089/scd.2011.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niyibizi C, Eyre DR. Structural Characteristics Of Cross-Linking Sites In Type-V Collagen Of Bone - Chain Specificities And Heterotypic Links To Type-I Collagen. Eur J Biochem. 1994;224:943–950. doi: 10.1111/j.1432-1033.1994.00943.x. [DOI] [PubMed] [Google Scholar]
- 53.Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sorensen ES, et al. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcified Tissue Int. 2005;77:45–54. doi: 10.1007/s00223-004-1288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin - a Possible Anchor Of Osteoclasts To Bone. P Natl Acad Sci USA. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Nam HK, Campbell C, Gasque KCD, Millan JL, Hatch NE. Tissue-nonspecific alkaline phosphatase deficiency causes abnormal craniofacial bone development in the Alpl(−/−) mouse model of infantile hypophosphatasia. Bone. 2014;67:81–94. doi: 10.1016/j.bone.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puchacz E, Lian JB, Stein GS, Wozney J, Huebner K, Croce C. Chromosomal Localization Of the Human Osteocalcin Gene. Endocrinology. 1989;124:2648–2650. doi: 10.1210/endo-124-5-2648. [DOI] [PubMed] [Google Scholar]
- 57.Khan SN, Fraser JF, Sandhu HS, Cammisa FP, Girardi FP, Lane JM. Use of osteopromotive growth factors, demineralized bone matrix, and ceramics to enhance spinal fusion. J Am Acad Orthop Sur. 2005;13:129–137. doi: 10.5435/00124635-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Deng Y, Lin XS, Zheng Z, Deng JG, Chen JC, Ma H, et al. Poly(hydroxybutyrate-co-hydroxyhexanoate) promoted production of extracellular matrix of articular cartilage chondrocytes in vitro. Biomaterials. 2003;24:4273–4281. doi: 10.1016/s0142-9612(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 59.Kaveh K, Ibrahim R, Abu Bakar MZ, Ibrahim TA. Mesenchymal Stem Cells, Osteogenic Lineage and Bone Tissue Engineering: A Review. J Anim Vet Adv. 2011;10:2317–2330. [Google Scholar]
- 60.Gao C, Seuntjens J, Kaufman GN, Tran-Khanh N, Butler A, Li AL, et al. Mesenchymal stem cell transplantation to promote bone healing. J Orthop Res. 2012;30:1183–1189. doi: 10.1002/jor.22028. [DOI] [PubMed] [Google Scholar]
- 61.de Rham C, Villard J. How to cross immunogenetic hurdles to human embryonic stem cell transplantation. Semin Immunopathol. 2011;33:525–534. doi: 10.1007/s00281-011-0262-z. [DOI] [PubMed] [Google Scholar]
- 62.Santiago JA, Pogemiller R, Ogle BM. Heterogeneous differentiation of human mesenchymal stem cells in response to extended culture in extracellular matrices. Tissue Eng Part A. 2009;15:3911–3922. doi: 10.1089/ten.tea.2008.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratajczak MZ, Kucia M, Majka M, Reca R, Ratajczak J. Heterogeneous populations of bone marrow stem cells--are we spotting on the same cells from the different angles? Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2004;42:139–146. [PubMed] [Google Scholar]
- 64.Deehan DJ, Dowen DJ, Sprowson AP, Ferguson LC, Prathalingam NS, Isaacs JD, et al. Differential Release of Heterogeneous Human Mesenchymal Stem Cell Populations from Haemarthrotic Traumatic Knee Injury. American Journal of Stem Cell Research. 2012;1:1–8. [Google Scholar]
- 65.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munavalli GS, Smith S, Maslowski JM, Weiss RA. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2013;39:1226–1236. doi: 10.1111/dsu.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taranger CK, Noer A, Sorensen AL, Hakelien AM, Boquest AC, Collas P. Induction of dedifferentiation, genomewide transcriptional programming, and epigenetic reprogramming by extracts of carcinoma and embryonic stem cells. Molecular biology of the Cell. 2005;16:5719–5735. doi: 10.1091/mbc.E05-06-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu XQ, Pan XH, Wang W, Chen Q, Pang RQ, Cai XM, et al. Transient in vitro epigenetic reprogramming of skin fibroblasts into multipotent cells. Biomaterials. 2010;31:2779–2787. doi: 10.1016/j.biomaterials.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kole D, Ambady S, Page RL, Dominko T. Maintenance of Multipotency in Human Dermal Fibroblasts Treated with Xenopus laevis Egg Extract Requires Exogenous Fibroblast Growth Factor-2. Cellular reprogramming. 2014;16:18–28. doi: 10.1089/cell.2013.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madonna R. Human-Induced Pluripotent Stem Cells: In Quest of Clinical Applications. Mol Biotechnol. 2012;52:193–203. doi: 10.1007/s12033-012-9504-0. [DOI] [PubMed] [Google Scholar]
- 72.Urist MR. Bone - Formation by Autoinduction. Science. 1965;150:893. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 73.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. The spine journal : official journal of the North American Spine Society. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 74.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) The spine journal : official journal of the North American Spine Society. 2008;8:1011–1018. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 75.Willems E, Leyns L. Patterning of mouse embryonic stem cell-derived pan-mesoderm by Activin A/Nodal and Bmp4 signaling requires Fibroblast Growth Factor activity. Differentiation. 2008;76:745–759. doi: 10.1111/j.1432-0436.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 76.Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;(38 Suppl 1):S11–S25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 77.Augello A, De Bari C. The Regulation of Differentiation in Mesenchymal Stem Cells. Human gene therapy. 2010;21:1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 78.Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomedical Sci & Eng. 2013:6. doi: 10.4236/jbise.2013.68A1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levine AJ, Brivanlou AH. GDF3, a BMP inhibitor, regulates cell fate in stem cells and early embryos. Development. 1992 Jan;114(1):253–259. doi: 10.1242/dev.02192. 2006;133 209–16. [DOI] [PubMed] [Google Scholar]
- 80.Levine AJ, Levine ZJ, Brivanlou AH. GDF3 is a BMP inhibitor that can activate Nodal signaling only at very high doses. Developmental Biology. 2009;325:43–48. doi: 10.1016/j.ydbio.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peerani R, Rao BM, Bauwens C, Yin T, Wood GA, Nagy A, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 1989 Sep;8(9):2601–2604. doi: 10.1038/sj.emboj.7601896. 2007;26:4744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dy P, Wang WH, Bhattaram P, Wang QQ, Wang L, Ballock RT, et al. Sox9 Directs Hypertrophic Maturation and Blocks Osteoblast Differentiation of Growth Plate Chondrocytes. Dev Cell. 2012;22:597–609. doi: 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xue R, Li JY, Yeh Y, Yang L, Chien S. Effects of matrix elasticity and cell density on human mesenchymal stem cells differentiation. J Orthop Res. 2013;31:1360–1365. doi: 10.1002/jor.22374. [DOI] [PubMed] [Google Scholar]
- 84.Luo F, Hou TY, Zhang ZH, Xie Z, Wu XH, Xu JZ. Effects of initial cell density and hydrodynamic culture on osteogenic activity of tissue-engineered bone grafts. PLoS One. 2013;8:e53697. doi: 10.1371/journal.pone.0053697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, et al. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110:8680–8685. doi: 10.1073/pnas.1301190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouet G, Cruel M, Laurent C, Vico L, Malaval L, Marchat D. Validation of an in vitro 3D bone culture model with perfused and mechanically stressed ceramic scaffold. Eur Cell Mater. 2015;29:250–266. doi: 10.22203/ecm.v029a19. discussion 66–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.