Abstract
Among the debilitating diseases, neurological related diseases are the most challenging ones to be treated using cell replacement therapies. Recently, dental pulp stem cells (SHED) were found to be most suitable cell choice for neurological related diseases as evidenced with many preclinical studies. To enhance the neurological potential of SHED, we recapitulated one of the pharmacological therapeutic tools in cell replacement treatment, we pre-conditioned dental pulp stem cells (SHED) with culture medium of ReNCell VM, an immortalized neuron progenitor cell, prior to neurogenesis induction and investigated whether this practice enhances their neurogenesis potential especially towards dopaminergic neurons. We hypothesed that the integration of pharmacological practices such as co-administration of various drugs, a wide range of doses and duration as well as pre-conditioning into cell replacement may enhance the efficacy of stem cell therapy. In particular, pre-conditioning is shown to be involved in the protective effect from some membrano-tropic drugs, thereby improving the resistance of cell structures and homing capabilities. We found that cells pre-treated with ReNCell VM conditioned medium displayed bipolar structures with extensive branches resembling putative dopaminergic neurons as compared to non-treated cells. Furthermore, many neuronal related markers such as NES, NR4A2, MSI1, and TH were highly expressed (fold changes > 2; p < 0.05) in pre-treated cells. Similar observations were detected at the protein level. The results demonstrate for the first time that SHED pre-conditioning enhances neurological potential and we suggest that cells should be primed to their respective environment prior to transplantation.
Keywords: Neural progenitor cells, Mesenchymal stem cells, Neurogenesis, Tyrosine hydroxylase, Dopamine acetyl-transferase
Introduction
Stem cells (SCs) refer to a group of cells which are capable of self-renewal and able to differentiate into many different types of tissue. Traditionally, these cells are found in bone marrow-mesenchymal stem cells (BM-MSCs) and most characterized SCs up to date (Knight and Hankenson 2013). However its invasive procurement techniques and low cell yield when culturing them in vitro have called for the search for alternative cell source. As the consequences, SCs are now found nearly in all organs and tissues of the human body. Among these, the tooth tissues namely the pulp offers an excellent reservoir of SCs. We have previously shown that these cells under appropriate cue can be differentiated into beta-like cells (Govindasamy et al. 2010a), adipocytes (Govindasamy et al. 2010b), chondrocytes and osteocytes (Govindasamy et al. 2010a). Likewise, others also have reported the ability of dental pulp stem cells from deciduous teeth (SHED) into cardiac-like (Samper et al. 2013) and hepatic like cells (Ishkitiev et al. 2012). As such, SHED are now being investigated as closely relevant cell source to tackle issues related to neurons owing to their inborn ectodermal origin.
Among the debilitating diseases known to affect human life severely, neurodegenerative-related issues such as Parkinsons, Alzheimers, Huntingtons, stroke and anoxic brain injury possess a great challenge to treat due to inadequate knowledge about the disease etiology and pathogenesis. Although these poorly managed diseases are worthy targets for cell replacement therapy, many factors especially whether the SCs are able to differentiate into cells of interest of a neuronal region need to be taken into consideration. This is because arrays of neuronal subtypes are available such as motor neurons, glial cells (oligodendrocytes and astrocytes), dopaminergic, Schwann cells and etc. To ascertain this notion, we have selected dopaminergic differentiation as a study model and investigated whether or not SHED could be able to differentiate into these cell lines.
The differentiation into dopaminergic neuron can be mediated in different ways. Among them are the usage of feeder layers such as MS5 cells (Hong et al. 2008) and mice PA6 cells (Kawasaki et al. 2002), as well as a wide range of mid-brain growth factors such as fibroblasts growth factor 8 (FGF-8), glial derived neurotrophic factor (GDNF), and brain derived neurotrophic factor (BDNF) (Daadi et al. 2012). Here, we have chosen pre-conditioning method to enhance the differentiation potential of SHED into dopaminergic-like cells.
Medium pre-conditioning refers to a technique that allows cells to grow in culture medium consisting of certain chemicals and growth factors with the aim of priming them according to intended micro-environment (Wu et al. 2011). Previous studies have reported the success of this technique in various aspects such as bone regeneration (Lu et al. 2013), immunomodulatory potency (Plotnikov et al. 2013), renal ischemic injury amelioration (Masoud et al. 2012), infarcted myocardium restoration (Choudhery et al. 2012) and many more. On top of this, choosing proper cues is another vital factor to consider in order to maximize the pre-conditioning effect.
ReNcell VM is an immortalized neuronal progenitor cell line which is being applied in various studies related to neuron development (Mußmann et al. 2014; Chaudhry and Ahmed 2013; Hernández-Benítez et al. 2013; Jaeger et al. 2013). The medium of this cell line is chosen to serve as mediator to deliver appropriate signals to enhance the induction process. Having said thus we initiated this study to understand the end result of SHED pre-condition with ReNcell VM medium in the differentiation into dopaminergic neurons.
Materials and methods
Sample population
The study was conducted using samples isolated from paediatric donors. Prior to the commencement of subject recruitment, approval for the study was acquired from the Medical Ethics Committee, Faculty of Dentistry, University of Malaya (Ethics approval number: DF CO1107/0066[L]).
Pulp collection and isolation of cells
Stem cell cultures derived from healthy deciduous donors (n = 3) were established as previously described by our group (Govindasamy et al. 2010a, b). Briefly, root surfaces were cleaned with Povidone-iodine (Sigma-Aldrich, St. Louis, MO, USA; http://www.sigmaaldrich.com), and the pulp was extirpated within two hours post-extraction, and processed. The pulp tissue was minced into smaller fragments prior to digestion in a solution of 3 mg/mL collagenase type I (Gibco, Grand Island, NY, http://www.invitrogen.com) for 40 min at 37 °C. After neutralization with 10 % FBS, the cells were centrifuged and seeded in culture flasks.
Cells were cultured in identical culture condition, namely in T75 cm2 culture flasks (BD Pharmingen, San Diego CA, USA; http://www.bdbiosciences.com) with culture medium containing 1× KO-DMEM, 200 U/mL and 200 µg/mL of penicillin/streptomycin (Invitrogen); 0.01× Glutamax (Invitrogen) and 10 % FBS with humidified atmosphere of 95 % of air and 5 % of CO2 at 37 °C, as well as cell seeding of 1,000 cell/cm2. Non-adherent cells were removed 48 h after initial plating. The medium was replaced every three days until the cells reached 80–90 % confluency.
Growth kinetics
The proliferation rate of SHED was determined by plating 5,000 cells/cm2 into separate T25 cm2 culture flask (BD Pharmingen). There were three replicates for each passage. SHED were detached by trypsinization after reaching 90 % confluency. Cells were counted and assessed for viability using trypan blue dye exclusion before the next sub-culture. Cells were re-plated for subsequent sub-culture, and a total of five sub-cultures were studied in this experiment. Growth kinetics was analyzed by calculating population doubling (PD) time. The PD time was obtained using:
NI is the inoculums cell number, NH is the cell harvest number and t is the time of the culture (in h).
Senescence assay
SHED were tested for senescence-associated β-galactosidase (SA-β-gal) activity at sub-culture 3 (SC 3) with the SA-β-gal staining kit (Sigma-Aldrich) and used according to the manufacture’s instruction. Briefly, SHED were washed twice with DPBS (−Ca2+, −Mg2+, Invitrogen) and incubated with 1× fixative solution for 15 min at room temperature. Subsequently, the fixed cells were re-washed using 2 mL of DPBS (−Ca2+, −Mg2+, Invitrogen) and stained with 1 mL of the staining solution mixture overnight at 37 °C in a drying incubator. The development of the blue colour was observed under a phase-contrast microscope and the quantitative analyses of the SA-β-gal staining was done by counting the percentage of blue-stained cells that represent senescence cells in the selected field of each sample.
Three-lineage differentiation
Mulitipotency of SHED was assessed by differentiating them into adipocytes, chondrocytes, and osteocytes as described previously (Yazid et al. 2014; Govindasamy et al. 2011). Briefly, adipogenic differentiation was initiated by inducing the cells with 200 mM indomethacin, 0.5 mM 3-isobutyl-1-methyxanthine (IMBX), 10 mg/mL insulin and 1 mM dexamethasone (all reagents from Sigma-Aldrich). Lipid droplets in the adipocytes generated were visualized by staining with Red Oil staining (Sigma-Aldrich). For chondrogenic differentiation, the cells were supplemented with 1 % ITS-1 (Sigma-Aldrich), 50 mM l-ascorbic acid-2 phosphate, 55 mM sodium pyruvate (Invitrogen), 25 mM l-proline (Sigma-Aldrich) and 10 ng/mL transforming growth factor-beta 3 (TGF-β3; Sigma-Aldrich). Assessment of proteoglycan accumulation was visualized by Alzarin Blue staining (Sigma-Aldrich). The osteogenic differentiation was stimulated in a 3-week culture with 10−7 M dexamethasone, 10 mM β-glycerol phosphate (Fluka, Buchs, Switzerland) and 100 mM l-ascorbic acid-2 phosphate. Assessment of calcium accumulation was visualized by Von Kossa staining (Sigma-Aldrich). Important genes related to fat, cartilage and bone development were also checked and the primers are listed in Table 1.
Table 1.
List of genes with primer sequence and their product size
| Gene name | Forward sequence (5′–3′) | Reverse sequence (5′–3′) | Base pair size |
|---|---|---|---|
| RUNX2 | GTCACTGTGCTGAAGAGGCT | GTCACTGTGCTGAAGAGGCT | 119 |
| BGLAP | CAGAGGTGCAGCCTTTGTGTC | TCACAGTCCGGATTGAGCTCA | 150 |
| PPARγ2 | ACAGCAAACCCCTATTCCATGCTGT | TCCCAAAGTTGGTGGGCCAGAA | 159 |
| LPL | TGGACTGGCTGTCACGGGCT | GCCAGCAGCATGGGCTCCAA | 167 |
| ACAN | AGGGCGAGTGGAATGATGTT | GGTGGCTGTGCCCTTTTTAC | 68 |
| COL2A1 | CTGCAAAATAAAATCTCGGTGTTCT | GGGCATTTGACTCACACCAGT | 101 |
| 18s rRNA | CGGCTACCATCCAAGGAA | GCTGGAATTACCGCGGCT | 186 |
| POU5F1 | CGACCATCTGCCGCTTTGAG | CCCCCTGTCCCCCATTCCTA | 573 |
| POU5F1 (qPCR) | TCCCGAATGGAAAGGGGAGA | GGCTGAATACCTTCCCAAATAGA | 209 |
| SOX2 | CCCCCGGCGGCAATAGCA | TCGGCGCCGGGGAGATACAT | 448 |
| SOX2 (qPCR) | GGACAGTTACGCGCACATGA | AGCCGTTCATGTAGGTCTGC | 188 |
| REXO1 | TCGCTGAGCTGAAACAAATG | CCCTTCTTGAAGGTTTACAC | 170 |
| ABCG2 | GTTTATCCGTGGTGTGTCTGG | CTGAGCTATAGAGGCCTGGG | 652 |
| ABCG2 (qPCR) | CTGAGCTCGTCCCCTGGAT | CTTTTGAGTGGGCACAGCAC | 182 |
| NES | CAGCGTTGGAACAGAGGTTGG | TGGCACAGGTGTCTCAAGGGTAG | 389 |
| NES (qPCR) | GTAGCTCCCAGAGAGGGGAA | CTCTAGAGGGCCAGGGACTT | 206 |
| NANOG | ATGCCTCACACGGAGACTGT | AGGGCTGTCCTGAATAAGCA | 66 |
| NR4A2 | CGGACAGCAGTCCTCCATTAAGGT | CTGAAATCGGCAGTACTGACAGCG | 712 |
| NR4A2 (qPCR)CR) | CGCCTGTAACTCGGCTGAA | AGTGTTGGTGAGGTCCATGC | 169 |
| PAX6 | ATGAACAGTCAGCCAATGGG | CACACCAGGGGAAATGAGTC | 63 |
| MSI1 | CAGCCAAAGGAGGTGATGTC | CGCTGATGTAACTGCTGACC | 451 |
| MSI1 (qPCR) | TGACCAAGAGATCCAGGGGT | AGTCACCATCTTGGGCTGTG | 152 |
| GFAP | GGCCCGCCACTTGCAGGAGTACCAGG | CTTCTGCTCGGGCCCCTCATGAGACG | 328 |
| GAFP (qPCR) | GTCAGAAGGCCACCTCAAGA | GAGGCGGAGCAACTATCCTG | 177 |
| TUBB3 | GCGAGATGTACGAAGACGAC | TTTAGACACTGCTGGCTTCG | 115 |
| TH | TCATCACCTGGTCACCAAGTT | GGTCGCCGTGCCTGTACT | 125 |
| DAT | CTGGTGTCTGGAAGATCTGC | AGCTGTCTCCACTGGAGTCA | 219 |
| DAT (qPCR) | AAAGTCCTTTCCCGATGCGT | ATACCAGGACCCCCATCCTC | 111 |
| NCAM | CAGTCCGTCACCCTGGTGTGCGATGC | CAGAGTCTGGGGTCACCTCCAGATAGC | 727 |
| NCAM (qPCR) | TCTGCTAGCTCGTCTACCCC | AGCTTAGGTGCACTGGGTTC | 110 |
Neuronal induction
SHED were subjected into neuronal induction at SC 3 by using chemically-defined media as described by Wang et al. (2010). Briefly, SHED were seeded at 1500 cells/cm2 in two separate groups: the first group for undergoing pre-conditioning via co-culturing with conditioned medium (CM) of an immortalized cell line (ReNCell VM) for 7 days while the other group for undergoing direct differentiation with Neuronal Medium A consisting of Neurobasal A, B27 supplement, 20 ng/mL basic Fibroblast Growth Factor (bFGF), and 20 ng/mL Epidermal Growth Factor (EGF) for 9 days. The first group was exposed to Neuronal Medium A upon 7 days exposure to CM. After both groups have been accordingly exposed to Neuronal Medium A, they were then treated in the second phase of neuronal differentiation (Neuronal Medium B) which consisted of Neurobasal A, 200 ng/mL sonic hedgehog (SHH), 100 ng/mL Fibroblast Growth Factor 8 (FGF8), 10 ng/mL brain-derived neurotrophic factor (BDNF) and 10 μM forskolin (Sigma-Aldrich) for 7 days. The CM was prepared freshly by collecting the medium after culturing ReNCell VM in its culture medium (Merck) for 7 days and removal of cell debris was performed via centrifugation force (1,500 rpm/6 min). All other chemicals were purchased from Invitrogen unless stated otherwise.
Semi-quantitative and quantitative gene expression via polymerase chain reaction (PCR)
PCR was performed in 0.2 mL tubes (Eppendorf) with a final volume of 12.5 μL as previously described (Govindasamy et al. 2010a). In brief, RNA isolation using Trizol (Invitrogen) was conducted at the end of induction period from a T25 culture flask with seeding density of 1,500 cells/cm2. RNA from cells was then subjected to DNase I treatment (Ambion, Foster City, CA, USA) to eliminate any traces of DNA contamination. cDNA amplification was performed in a thermocycler (Applied Biosystems, Foster City, CA, USA) for 35 cycles using Taq polymerase supplied with KCl buffer and 1.5 mM MgCl2 (Invitrogen) at 94 °C/1 min, 58 °C/30 s and 72 °C/1 min. PCR products were resolved on 1.5 % agarose (Invitrogen) gel in 1× Tris borate–EDTA buffer. The expression levels of the genes via real-time PCR were quantified in duplicates, using SYBR Green Master Mix (Applied Biosystems). PCR reactions were carried out on AB ViiA7 (Applied Biosystems), and the results were analyzed using a software called SDS v 2.1. Gene expressions were analyzed via comparative CT Method (ΔΔCT) and were normalized to 18s rRNA. The gene expression was compared against undifferentiated cell as control group and the primer sequences are listed in Table 1.
Immunofluorescent analysis
Differentiated cells which were seeded at 1,500 cells/cm2 in four-well chamber slides (BD Falcon) were fixed for 20 min in 4 % ice cold paraformaldehyde, treated with 0.1 % Triton-X for optimal penetration of cell membranes, and incubated at room temperature (RT) in a blocking solution (0.5 % BSA; Sigma-Aldrich) for 30 min. Primary antibodies [Oligodendrocyte marker (mouse, Abcam, Cambridge, UK), Musashi 1 (rabbit, Abcam), beta-tubulin III (mouse, Millipore, Billerica, MA, USA), neurofilament (mouse, Upstate, Lake Placid, NY, USA), Microtubule-associated protein 2 (MAP2) (rabbit, Abcam), tyrosine hydroxylase (TH) (rabbit, Abcam) with dilution of 1:400 for all] were incubated overnight at 4 °C, washed with Dulbecco’s Phosphate Buffer Saline (DPBS; Invitrogen), and then incubated with secondary antibodies (either fluorescein isothiocyanate [FITC]-conjugated IgG against mouse/rabbit antigen or rhodamine-conjugated IgG against rabbit antigen) (all from Abcam) at RT for 90 min. Slides were counterstained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI, Chemicon, Temecula, CA, USA) for 5 min. Fluorescent images were captured by means of a Olympus BX63 microscope (Olympus, Tokyo, Japan, http://www.olympus.com).
Statistical analysis
Data are presented as mean ± standard deviation. The descriptive statistical tests were performed using the software SPSS for Windows (version 11.0, SPSS Predictive Analytics, Chicago, IL and http://www.spss.com). Homogeneity of variance and normality were also tested. The data were analyzed using two-way analysis of variance. Tukey’s post hoc multiple comparison tests were carried out to determine the differences between groups. The significance level was set at p = 0.05.
Results
Basic characterization of dental pulp stem cells from deciduous teeth
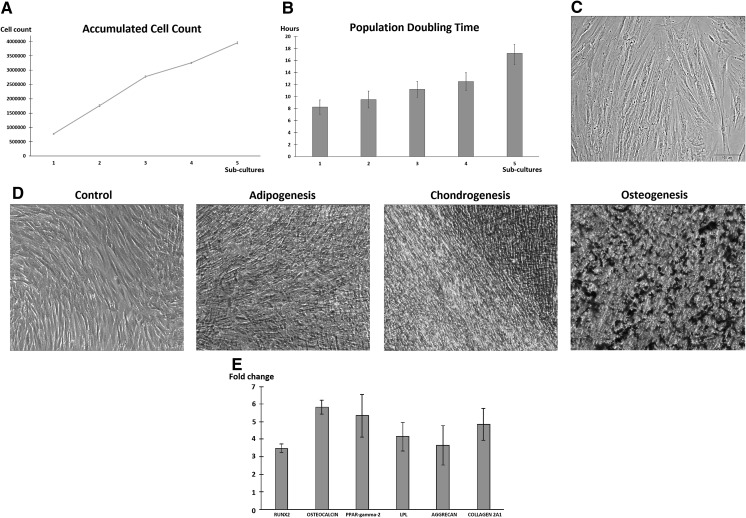
The morphological features of cells were fibroblastic throughout the culture period. In terms of growth analysis, the cumulative cell count presented an increase by almost four-fold at SC5 as compared to SC1. In addition, growth kinetics by means of PDT were also observed whereby two-fold changes in terms of hours was recorded at SC5 as compared to SC1 (Fig. 1B). The presence of senescent cells was also observed via beta-galactosidase activity and was shown to be 2.7 % indicating a reduced aging process to have taken place in SHED (Fig. 1C). The cells were also subjected to some mesoderm differentiations in which it were able to produce oil droplets (adipogenesis), forming networks of proteoglycan (chondrogenesis) as well as accumulation of black calcium precipitate (osteogenesis) upon chemical stimulation (Fig. 1D).
Fig. 1.
A, B Accumulated cell count and population doubling time (PDT) over five subcultures to understand the growth kinetics of SHED. C Senescence assay to assess the activity of betagalactosidase in SHED at SC3. D Trilineage differentiation of SHED at SC3. Adipogenesis was assessed via Red Oil staining and oil droplets formation was observed. Furthermore, chondrogenesis was evaluated using Alzarin Blue staining with the formation of proteoglycan networks. In addition osteogenesis was measured using Von Kossa staining with the presence of black precipitate. All micrographs were taken at 10x magnification and scale bar = 100μm. E Real-time PCR was performed to detect presence of representative genes related to adipogenesis, chondrogenesis and osteogenesis
Induction of dental pulp stem cells from deciduous teeth into dopaminergic-like cells
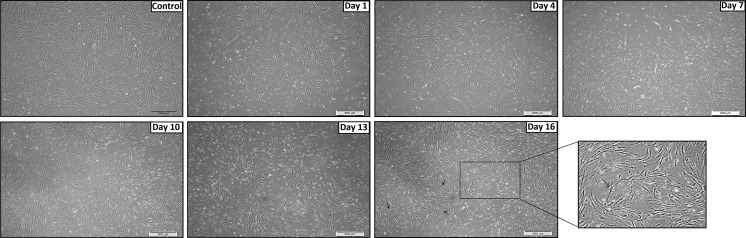
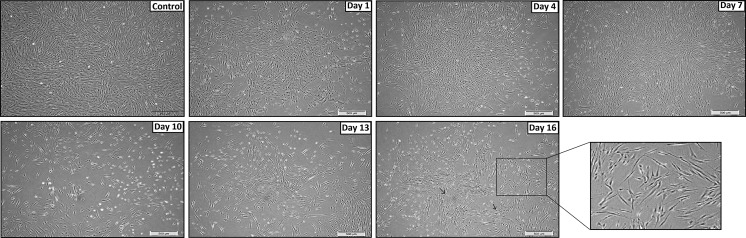
Upon co-cultivation of CM with SHED for 7 days, significant changes in terms of morphology of the cells were noted. (Fig. 2). Cells were shown to be more elongated and extensively branched as compared to control sample. As the induction period progresses, more extensions and cell branches were observed. Towards the end of induction (day 16), bipolar-like cells were witnessed with central nucleus while the axon-like structures were found to be stretched away from nucleus. Conversely without the pre-treatment with CM, SHED were seen to differentiate a little slowlier with reduced cell numbers (Fig. 3). During the induction period, SHED grew in colonies and formed cell cluster prior to day 10, surprisingly, from day 11 onwards the cells began to grow away from one another and started to elongate. Even though the cell number was reduced, the final morphology was still similar as pre-treated SHED whereby bipolar-like features were observed.
Fig. 2.
Induction of pre-conditioned SHED into dopaminergic neurons. Cells rapidly began to elongate and formed branches while some even form star-shaped cells up to day 10. Following day 10, cells were found to grow near each other and form ‘whirlpool’ shaped colonies up to day 16. Bipolar phenotypes were also observed in some of the cells. Micrographs were taken at 10x magnification and scale bar = 500 μm. The section of the day 16 panel was taken at 20x magnification. Control picture was taken on day 8 in serum-containing culture medium
Fig. 3.
Induction of SHED into dopaminergic neurons without pre-conditioning. Cells were growing in clusters in elongated form till day 7. From day 10 onwards, the cells began to form thin branches till day 16. Cell number was found to have been reduced throughout the study period. Micrographs were taken at 10x magnification and scale bar = 500 μm. The section of the day 16 panel was taken at 20x magnification. Control picture was taken on day 8 in serum-containing culture medium
Gene expression profile of dental pulp stem cells from deciduous teeth transformed into dopaminergic-like cells
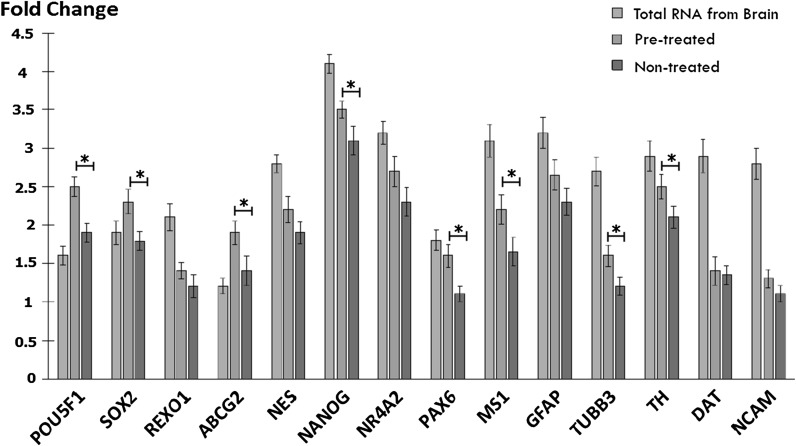
SHED from both conditions showed similar gene expression profile as described in Figs. 4 and 5. Pluripotent markers like OCT4, SOX2, NANOG, REX1, and ABCG2 were lightly present as compared to undifferentiated cells. Furthermore early and mid-neuronal genes like NESTIN, NURR1, PAX6, and MUSASHI1 were present in both conditions with an increased expression observed in pre-treated SHED. Likewise, mature neuronal markers namely glial-fibrillary acidic protein (GFAP), beta-tubulin, TH, dopamine active transporter (DAT), and neural cell adhesion molecule (NCAM) were expressed slightly higher in pre-treated as compared to non-treated SHED.
Fig. 4.
Detection of pluripotent as well as neuronal markers via semi-quantitative PCR. Lane A refers to undifferentiated SHED; Lane B denotes gene expression using total RNA from Brain; Lane C shows gene expression of pre-conditioned SHED and Lane D shows gene expression of SHED without preconditioning. Pluripotent markers (POU5F1, SOX2, REX01, and ABCG2) were highly expressed in undifferentiated cells (Lane A) whereas reduced expression was observed in Total RNA from brain (Lane B), pre-conditioned SHED (Lane C) as well as in SHED without pre-conditioning (Lane D). Early neuronal markers (NES, NANOG, NR4A2, PAX6, and MSl1) were present in both Lane C and Lane D with the former showing an increased expression. Similarly, mature neuronal markers (GFAP, beta-Tub, TH, DAT, and NCAM) were shown to present in both Lane C and Lane D with the former showing an increased expression
Fig. 5.
Detection of pluripotent as well as neuronal markers. The Ct value of genes were analyzed in the study using SYBR green-based qRT–PCR for SHED. Generally the higher a fold change value, the more copies are present in the specific sample. Total RNA from brain was used as positive control. Values are presented after normalization to 18s mRNA levels (* p < 0.05)
Protein expression analysis of dental pulp stem cells from deciduous teeth into dopaminergic-like cells
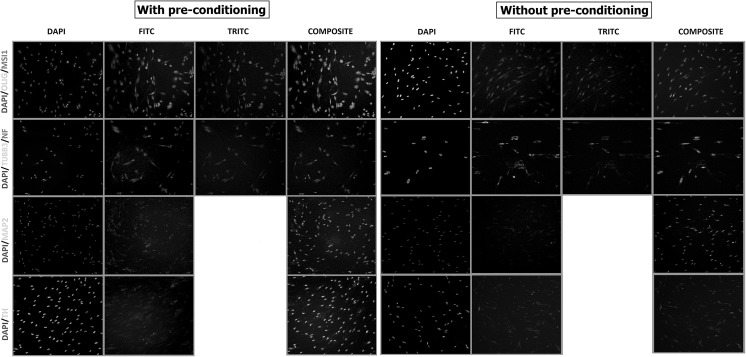
Further analysis by means of immunofluorescence technique revealed that pre-conditioned SHED expressed all markers namely beta-tubulin, neurofilament (NF), microtubule-associated-protein 2 (MAP2), oligodendrocyte (OLIG), Musashi (MSI1) and TH slightly more in comparison with those without pre-conditioning (Fig. 6).
Fig. 6.
Immunocytochemistry analysis revealing the presence of neuronal marker namely Oligodendrocyte transcription factor (OLIG), RNA-binding protein Musashi homolog 1 (MSI1), beta—tubulin III (b-TUB), neurofilament (NF), microtubule-associated protein 2 (MAP2), and tyrosine hydroxylase (TH) in differentiated SHED from both pre-conditioned with CM and without pre-conditioning, respectively. It should be noted that the protein expression in those SHED with pre-conditioning were qualitatively higher as compared to those without pre-conditioning
Discussion
In this study, our results were concurrent with previous reports whereby cells were of fibroblastic phenotype and could be expanded in culture flasks (Yazid et al. 2014; Abu Kasim et al. 2012; Govindasamy et al. 2010a). On top of that SHED were shown to have decent outcome in terms of cell count, PDT as well as senescence assay. This specify that cells in optimal state and hence suitable for down-stream works (Sethe et al. 2006). Further, despite being a cell of neural crest origin (Kanafi et al. 2014), the ability of SHED to differentiate into three types of cell lineages indicates plasticity nature (Hass et al. 2011).
Moving on to the dopaminergic-like cells induction, both culture conditions (with and without pre-conditioning treatment) revealed apparent phenotypic structure of dopaminergic-like cells as previously reported (Kanafi et al. 2014; Govindasamy et al. 2010a; Wang et al. 2010) with SHED pre-treated with CM showed a better morphology. It has been reported previously that pre-conditioning improves cell differentiation to a greater extent (Wu et al. 2012) and this is due to the presence of numerous cytokines and neurotrophic factors within the microenvironment (Plotnikov et al. 2013). These factors could serve as internal signaling molecules to guide SHED to differentiate into dopaminergic neurons accordingly.
Furthermore, gene expression profiling showed that pre-conditioned SHED expressed higher early neuronal markers as compared to those without pre-conditioning. Overall, the expression of pluripotent indicators such as POU5F1, SOX2, NANOG, REX01, and ABCG2 were decreased as compared to undifferentiated cells indicating a commitment of SHED towards neuronal-lineage (Chen and Dent 2014). Pre-conditioned SHED have shown lower expression of the aforesaid genes as compared to those without pre-conditioning. This perhaps could be due to more proportion of cells have fully differentiated into dopaminergic neurons thus having low number of stem cells expressing pluripotent indicators.
As distinctive neurogenesis takes place, early and mid-neuronal genes like NES MS1, NR4A2 and PAX6 begin to show up in both conditions with more expression being observed in pre-treated SHED. It was reported previously that NES is well known for its role in inducing neurogenesis (Lagace et al. 2007). Furthermore MS1 is involved in stem cell self-renewal and asymmetric cell division which is highly correlated with proliferation, survival and neurogenesis (Pozniak and Pleasure 2006). Additionally NR4A2 was reported as one of major key regulators in dopaminergic neuron formation (Hong et al. 2014). PAX6 gene moreover is responsible in survival of immature neurons via CREB-signalling (Faigle and Song 2013). Collectively these molecular cues have systematically coaxed SHED from their pluripotent stage to differentiate into mid-neuronal phase.
As the neurogenesis process began to develop, mature neuronal markers namely GFAP, TUBB3, TH, DAT, and NCAM were also seen to be obviously present with higher expression being observed in pre-conditioned SHED in comparison to those without pre-conditioning. GFAP has been reported as the major indicator related to intermediate filament of mature astrocytes in the mammalian central nervous system (Hagemann et al. 2013). Moreover the presence of both NCAM and beta tubulin genes indicated the incidence of mature neurons especially those of granule cell layer and external plexiform layer which are typical in olfactory bulb granule cells (Lledo et al. 2006). TH and DAT are mutually vital key indicators for fully functional dopaminergic neurons (Agoston et al. 2014; Kim et al. 2007). These genes altogether have shown the presence of mature neuronal markers which evolved from their previous mid-neuronal phase. Similarly protein expression profile by means of immunofluorescence revealed that pre-conditioned SHED having more expression as compared to those without pre-conditioning. On top of indicating the presence of the said protein in different stages of neurogenesis, another notion whereby CM has influenced all these features in positive ways can be said as well.
Besides, we have shown in this study the potentiality of SHED to be induced into dopaminergic neurons. These neurons are very important predominantly in Parkinson’s disease (Wang et al. 2010). Perhaps CM can be taken to our advantage provided that we have understood enough about it in order to find an answer for this neurodegenerative problem. It is believed that the cytokines and small molecules which are present in CM played a vital role in enhancing neurogenesis throughout the study period. A study has shown that glial conditioned medium (GCM) has a neuroprotective effect onto immortalized striatal neuronal progenitor cell lines (Ruiz et al. 2012). Not just that, the GCM was also able to reduce cell death, it increased cell survival, and reduced caspases fragmentation (Whone et al. 2012) as well as accumulation of reactive oxygen species in addition to polyubiquitinated proteins (Neher et al. 2011). The neurotrophic factors that were present in GCM were believed to be responsible for such findings. Contrastingly, Horn and Bernardi (2011) have reported that the CM from MSC was apparently toxic to cell culture originated from hippocampus and this was due to the side effects of the small molecules present in the said CM. Further work revealed that the secreted cytokines from MSC triggered reactive species generation and neuroinflammation in organotypic cultures of hippocampus.
Despite these distinctions further understanding regarding CM is paramount since the mechanism behind this phenomenon is highly related to the small molecules. Secretome profiling of CM is required so that the components of CM can be fully described which will enhance our appreciation towards CM. This will further help us to translate this critical information in clinical settings involving regenerative medicine especially related to cellular transplantation. There are reports showing that in vivo studies which have shown positive outcome in animal models could not be replicated in humans (Ankrum et al. 2014; Skuk 2013).
Thus we speculate that cells could possibly be pre-conditioned according to their respective microenvironment prior to transplantation. Perhaps pre-conditioning would help prime the cells so that they could home themselves in target organ plus differentiate accordingly in the event of cellular transplantation. However other dynamics like procurement of CM, length of pre-conditioning time, immune-rejection and cell management approaches should be properly revised and tested for several times in various models to effectively establish this method for future purposes.
Acknowledgments
This work is part of research collaboration between Hygieia Innovation and the Faculty of Dentistry, University of Malaya. This work is supported by the University of Malaya, High Impact Research Grant, Ministry of Higher Education, Malaysia (Grant No. UM.C/HIR/MOHE/DENT/01).
Conflict of interest
Authors declared no conflict of interest.
References
- Abu Kasim NH, Govindasamy V, Gnanasegaran N, Musa S, Pradeep PJ, Srijaya TC, Aziz ZA. Unique molecular signatures influencing the biological function and fate of post-natal stem cells isolated from different sources. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1663. [DOI] [PubMed] [Google Scholar]
- Agoston Z, Heine P, Brill MS, Grebbin BM, Hau AC, Kallenborn-Gerhardt W, Schramm J, Götz M, Schulte D. Meis2 is a Pax6 co-factor in neurogenesis and dopaminergic periglomerular fate specification in the adult olfactory bulb. Development. 2014;141:28–38. doi: 10.1242/dev.097295. [DOI] [PubMed] [Google Scholar]
- Ankrum JA, Ong JF, Karp JM (2014) Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32:252–260. doi:10.1038/nbt.2816 [DOI] [PMC free article] [PubMed]
- Chaudhry ZL, Ahmed BY. Caspase-2 and caspase-8 trigger caspase-3 activation following 6-OHDA-induced stress in human dopaminergic neurons differentiated from ReNVM stem cells. Neurol Res. 2013;35:435–440. doi: 10.1179/1743132812Y.0000000135. [DOI] [PubMed] [Google Scholar]
- Chen T, Dent SY. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet. 2014;15:93–106. doi: 10.1038/nrg3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhery MS, Khan M, Mahmood R, Mohsin S, Akhtar S, Ali F, Khan SN, Riazuddin S. Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair-infarcted myocardium. J Cell Mol Med. 2012;16:2518–2529. doi: 10.1111/j.1582-4934.2012.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Grueter BA, Malenka RC, Redmond DE, Jr, Steinberg GK. Dopaminergic neurons from midbrain-specified human embryonic stem cell-derived neural stem cells engrafted in a monkey model of Parkinson’s disease. PLoS One. 2012;7:e41120. doi: 10.1371/journal.pone.0041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy V, Abdullah AN, Ronald VS, Musa S, Ab Aziz ZA, Zain RB, Totey S, Bhonde RR, Abu Kasim NH. Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. J Endod. 2010;36:1504–1515. doi: 10.1016/j.joen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Govindasamy V, Ronald VS, Totey S, Din SB, Mustafa WM, Totey S, Zakaria Z, Bhonde RR. Micro manipulation of culture niche permits long-term expansion of dental pulp stem cells—an economic and commercial angle. Vitro Cell Dev Biol Anim. 2010;46:764–773. doi: 10.1007/s11626-010-9332-0. [DOI] [PubMed] [Google Scholar]
- Govindasamy V, Ronald VS, Abdullah AN, Nathan KR. Differentiation of dental pulp stem cells into islet-like aggregates. J Dent Res. 2011;90:646–652. doi: 10.1177/0022034510396879. [DOI] [PubMed] [Google Scholar]
- Hagemann TL, Paylor R, Messing A. Deficits in adult neurogenesis, contextual fear conditioning, and spatial learning in a Gfap mutant mouse model of Alexander disease. J Neurosci. 2013;33:18698–18706. doi: 10.1523/JNEUROSCI.3693-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Benítez R, Vangipuram SD, Ramos-Mandujano G, Lyman WD, Pasantes-Morales H. Taurine enhances the growth of neural precursors derived from fetal human brain and promotes neuronal specification. Dev Neurosci. 2013;35:40–49. doi: 10.1159/000346900. [DOI] [PubMed] [Google Scholar]
- Hong S, Kang UJ, Isacson O, Kim KS. Neural precursors derived from human embryonic stem cells maintain long-term proliferation without losing the potential to differentiate into all three neural lineages, including dopaminergic neurons. J Neurochem. 2008;104:316–324. doi: 10.1111/j.1471-4159.2007.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Chung S, Leung K, Hwang I, Moon J, Kim KS. Functional roles of nurr1, pitx3, and lmx1a in neurogenesis and phenotype specification of dopamine neurons during in vitro differentiation of embryonic stem cells. Stem Cells Dev. 2014;23:477–487. doi: 10.1089/scd.2013.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn AP, Bernardi A, Luiz Frozza R, Grudzinski PB, Hoppe JB, de Souza LF, Chagastelles P, de Souza Wyse AT, Bernard EA, Battastini AM, Campos MM, Lenz G, Nardi NB, Salbego C. Mesenchymal stem cell-conditioned medium triggers neuroinflammation and reactive species generation in organotypic cultures of rat hippocampus. Stem Cells Dev. 2011;20:1171–1181. doi: 10.1089/scd.2010.0157. [DOI] [PubMed] [Google Scholar]
- Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Nakahara T, Ishikawa H, Mitev V, Haapasalo M. High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J Endod. 2012;38:475–480. doi: 10.1016/j.joen.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Jaeger A, Baake J, Weiss DG, Kriehuber R. Glycogen synthase kinase-3beta regulates differentiation-induced apoptosis of human neural progenitor cells. Int J Dev Neurosci. 2013;31:61–68. doi: 10.1016/j.ijdevneu.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Kanafi M, Majumdar D, Bhonde R, Datta I (2014) Midbrain cues dictate differentiation of human dental pulp stem cells towards functional dopaminergic neurons. J Cell Physiol 229:1369–1377. doi:10.1002/jcp.24570 [DOI] [PubMed]
- Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, Haruta M, Takahashi M, Yoshikawa K, Nishikawa SI. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci. 2002;99:1580–1585. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Sugimori M, Nakafuku M, Svendsen CN. Control of neurogenesis and tyrosine hydroxylase expression in neural progenitor cells through bHLH proteins and Nurr1. Exp Neurol. 2007;203:394–405. doi: 10.1016/j.expneurol.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Knight MN, Hankenson KD. Mesenchymal stem cells in bone regeneration. Adv Wound Care. 2013;2:306–316. doi: 10.1089/wound.2012.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, DiLeone RJ, Greer CA, Mandyam CD, Eisch AJ. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lu Z, Wang G, Dunstan CR, Chen Y, Lu WY, Davies B, Zreiqat H. Activation and promotion of adipose stem cells by tumour necrosis factor-α preconditioning for bone regeneration. J Cell Physiol. 2013;228:1737–1744. doi: 10.1002/jcp.24330. [DOI] [PubMed] [Google Scholar]
- Masoud MS, Anwar SS, Afzal MZ, Mehmood A, Khan SN, Riazuddin S. Pre-conditioned mesenchymal stem cells ameliorate renal ischemic injury in rats by augmented survival and engraftment. J Transl Med. 2012;10:243. doi: 10.1186/1479-5876-10-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mußmann C, Hübner R, Trilck M, Rolfs A, Frech MJ (2014) HES5 is as a key mediator of Wnt-3a induced neuronal differentiation. Stem Cells Dev 23:1328–1339 [DOI] [PubMed]
- Neher JJ, Brown GC, Kinsner-Ovaskainen A, Bal-Price A (2011) Inflammation and reactive oxygen/nitrogen species in glial/neuronal cultures. Cell Culture Tech. Humana Press, New Jersey, pp. 331–347
- Plotnikov EY, Pulkova NV, Pevzner IB, Zorova LD, Silachev DN, Morosanova MA, Sukhikh GT, Zorov DB. Inflammatory pre-conditioning of mesenchymal multipotent stromal cells improves their immunomodulatory potency in acute pyelonephritis in rats. Cytotherapy. 2013;15:679–689. doi: 10.1016/j.jcyt.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Pozniak CD, Pleasure SJ. Genetic control of hippocampal neurogenesis. Genome Biol. 2006;7:207. doi: 10.1186/gb-2006-7-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz C, Casarejos MJ, Gomez A, Solano R, de Yebenes JG, Mena MA (2012) Protection by glia-conditioned medium in a cell model of Huntington disease. PLoS Currents 4. doi:10.1371/4fbca54a2028b [DOI] [PMC free article] [PubMed]
- Samper E, Diez-Juan A, Montero J, Sepúlveda P. Cardiac cell therapy: boosting mesenchymal stem cells effects. Stem Cell Rev Rep. 2013;9:266–280. doi: 10.1007/s12015-012-9353-z. [DOI] [PubMed] [Google Scholar]
- Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Skuk D. Cell transplantation and “stem cell therapy” in the treatment of myopathies: many promises in mice, few realities in humans. ISRN Transplant. 2013;2013:25. doi: 10.5402/2013/582689. [DOI] [Google Scholar]
- Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010;19:1375–1383. doi: 10.1089/scd.2009.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whone AL, Kemp K, Sun M, Wilkins A, Scolding NJ. Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res. 2012;1431:86–96. doi: 10.1016/j.brainres.2011.10.038. [DOI] [PubMed] [Google Scholar]
- Wu KH, Mo XM, Han ZC, Zhou B. Cardiac cell therapy: pre-conditioning effects in cell-delivery strategies. Cytotherapy. 2011;14:260–266. doi: 10.3109/14653249.2011.643780. [DOI] [PubMed] [Google Scholar]
- Yazid FB, Gnanasegaran N, Kunasekaran W, Govindasamy V, Musa S (2014) Comparison of immunodulatory properties of dental pulp stem cells derived from healthy and inflamed teeth. Clin Oral Investig. doi:10.1007/s00784-014-1207-4 [DOI] [PubMed]