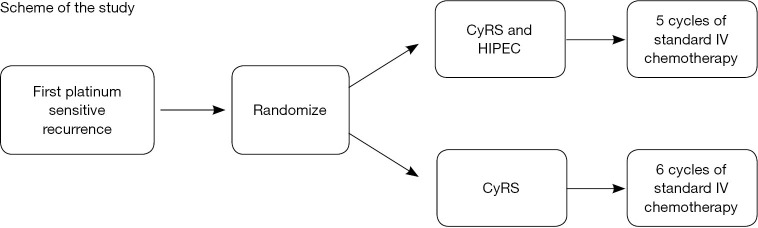
Figure 2.
A phase II randomized study for outcomes after secondary cytoreductive surgery with or without carboplatin HIPEC followed by systemic combination chemotherapy for recurrent platinum-sensitive ovarian, fallopian tube or primary peritoneal cancer. Shown is the schema for the study. After 1st platinum sensitive recurrence is identified the patients will be randomized intra-operatively after all eligibility criteria are met. Each arm includes 49 patients and post-operatively the patients will receive 5 cycles of standard IV chemotherapy in the HIPEC arm or 6 cycles in the non-HIPEC arm. Courtesy of Dennis Chi, MD (with permission) (https://clinicaltrials.gov/ct2/show/NCT01767675). CyRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy.