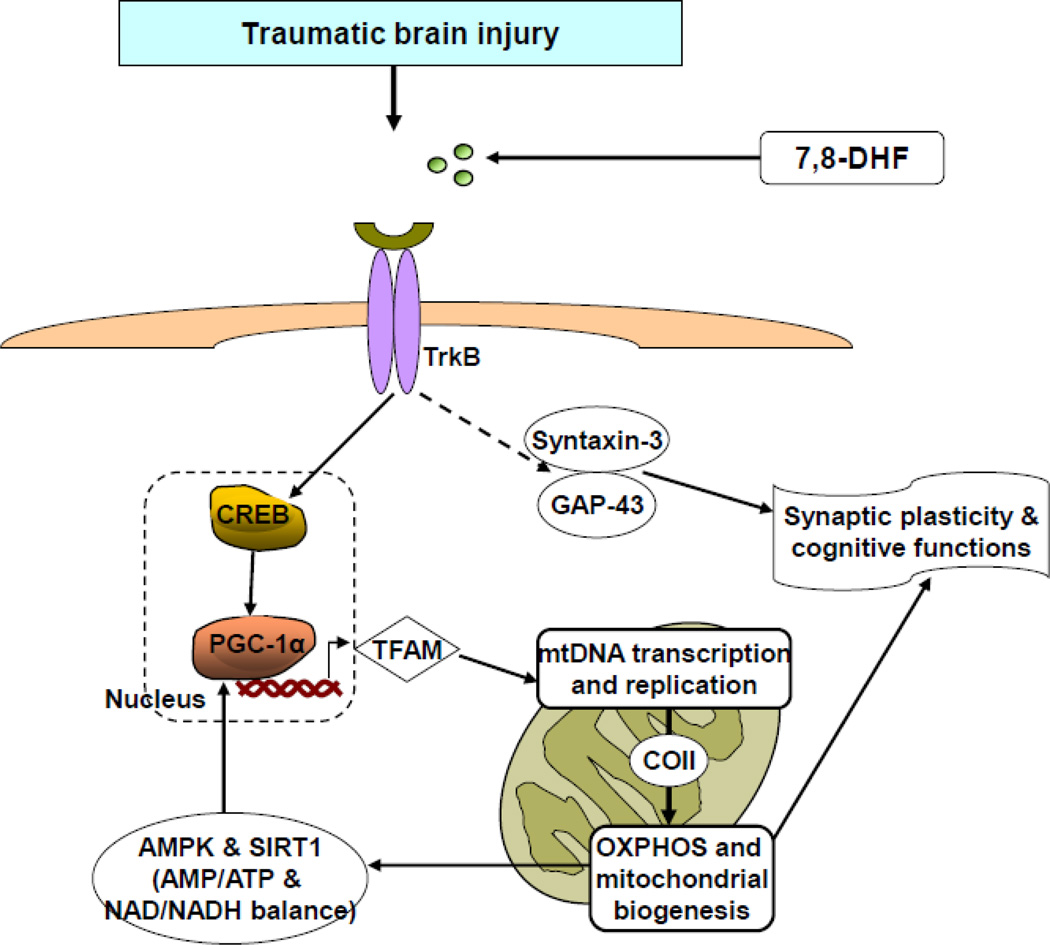
Fig. 8.
Proposed mechanism by which activation of BDNF receptor TrkB using 7,8-dihydroxyflavone (7,8-DHF), a small-molecule TrkB agonist, improves brain plasticity and mental health following traumatic brain injury (TBI). 7,8-DHF is a member of the flavonoid family that crosses the blood–brain barrier and activates the TrkB receptor similar to the BDNF. The treatment with 7,8-DHF restores the TBI induced disruption of TrkB signaling, which plays a pivotal action on mitochondrial biogenesis through metabolic activators such as PGC-1α. PGC-1α improves mitochondrial function by activating mitochondrial DNA (mtDNA) transcription, and mitochondrial proliferation through mitochondrial transcription factor A (TFAM). The mtDNA transcription leads to the synthesis of mitochondrial proteins including cytochrome c oxidase (COII), which is a key player for ATP production by oxidative phosphorylation (OXPHOS) in mitochondria. ATP and NAD are small molecules involved in all energy transactions in cells, and can be sensed by regulatory proteins, such as AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) respectively, which both have the capacity to activate PGC-1α. The alteration in AMPK-SIRT1-PGC-1α signaling pathway plays a critical role to regulate mitochondrial energy system, and is important for maintaining cognitive function and synaptic plasticity via CREB. Regulation of synaptic function by 7,8-DHF can also be mediated by syntaxin-3 and GAP-43. Overall, the diagram illustrates a mechanism by which the interplay between energy management and plasticity regulates neuronal function under homeostatic and injured conditions.