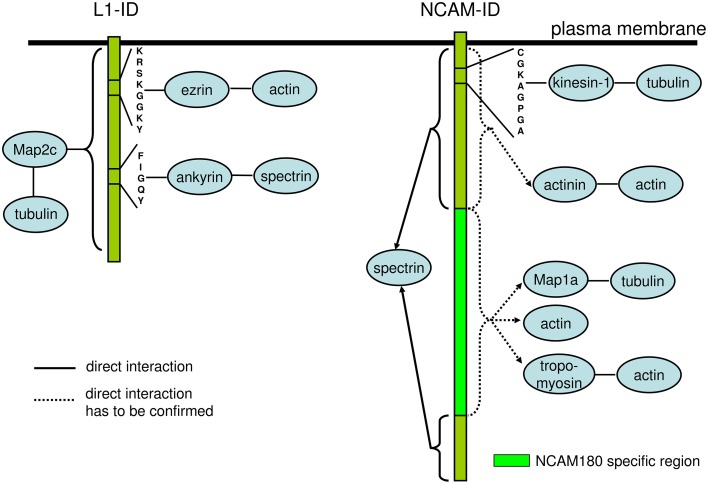
Figure 2.
Schematic diagram of the domains and motives within the intracellular domains of L1 and NCAM involved in interactions with the components of the cytoskeleton. The intracellular domain of L1 (L1-ID) comprises binding motives for ezrin and ankyrin linking L1-ID to the actin and spectrin, respectively. MAP2c links L1-ID to tubulin, however, the binding sequence for MAP2c within L1-ID is not known. The intracellular domain of NCAM (NCAM-ID) comprises a binding motive for kinesin-1, which can link it to tubulin. This motive is present in both transmembrane isoforms of NCAM, NCAM140, and NCAM180, which contain the same amino acid sequences except for the presence of the NCAM180 specific region encoded by exon 18 in NCAM180. Intracellular domains of both transmembrane isoforms of NCAM directly bind to spectrin. The exact binding motive for spectrin is not known and is probably present in amino acid sequences present in both NCAM isoforms. Actinin, MAP1a, tropomyosin and actin were isolated from the brain using NCAM-ID as bait, however, the direct binding of these proteins has not been analyzed and exact binding sequences are not known. Since actinin binds to intracellular domains of NCAM140 and NCAM180, while MAP1a and tropomyosin bind to the intracellular domain of NCAM180 only, the binding sequences for these proteins are located in regions either present in both NCAM isoforms or in NCAM180 specific region, respectively. See Table 1 for references.