Abstract
A copper-catalyzed borylation/ortho-cyanation/allyl group transfer cascade has been developed. Initiated by an unconventional copper-catalyzed electrophilic dearomatization, this process features a regio- and stereospecific 1,3-transposition of the allyl fragment enabled by an aromatization-driven Cope rearrangement. This method provides an effective means for the construction of adjacent tertiary and quaternary stereocenters with excellent diastereocontrol.
Keywords: copper, cyanation, C–C activation, dearomatization, sigmatropic rearrangement
Since its discovery in 1940,[1] the Cope rearrangement has been widely utilized as a powerful tool for the construction of complex molecular architectures.[2] Using the chair-like six-membered transition state as the dominant stereocontrol element, this venerable transformation allows for the efficient creation of well-defined stereochemical arrays in a predictable fashion. Due to the reversible nature of this sigmatropic rearrangement, the use of substrates featuring ring strain or possessing specialized substitution patterns (e.g., oxy-Cope)[2] represents the most commonly employed tactics to drive the reaction towards the desired rearranged products. In this context, the development of alternative strategies to facilitate the Cope rearrangement is pivotal to its further advancement as a synthetically useful transformation.
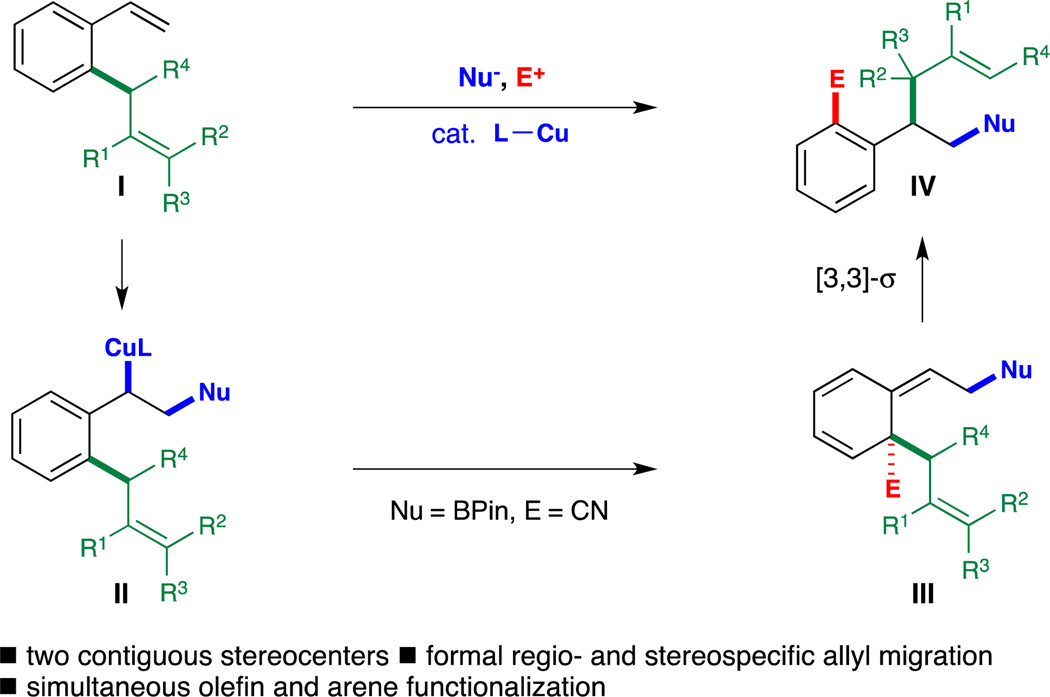
We previously reported that the capture of a vinylarene-derived benzylcopper species with an electrophilic cyanating reagent led to the highly selective formation of a dearomatized intermediate. [3,4] Very recently, the elegant work of Montgomery has expanded the scope of this transformation to simple styrenes.[5] A related borylative bromine recycling strategy was pioneered by Schomaker and coworkers,[6] which has been shown to proceed via a similar mechanism.[6d] Based on these precedents, we envisioned that the catalytic generation of a semibenzene intermediate (III) could serve as a generic platform for the development of a broad spectrum of novel rearrangement reactions, including the Cope rearrangement (Scheme 1). In contrast to conventionally applied strategies, this process utilizes aromatization as the crucial driving force for the rearrangement of these previously difficult-to-access dearomatized intermediates. As detailed in Scheme 1, interception of the transient benzylcopper intermediate II generated from 2-allylstyrene I with an electrophile would provide the dearomatized intermediate III. Enabled by the rearomatizing Cope rearrangement, the subsequent 1,3-transposition of the allyl unit would be expected to proceed in a completely regio- and stereospecific fashion, affording densely functionalized products bearing two adjacent stereocenters. Notably, the selective cleavage of the allyl fragment from an aromatic moiety and the subsequent allyl group shuttling achieved in this cascade remained unexplored in the context of bond activation chemistry.[7] We recognized that the realization of this proposed transformation would be nontrivial. The electrophilic cyanation of benzylcopper II would give rise to an unstable dearomatized intermediate III possessing an all-carbon quaternary center, and could thus be both thermodynamically and kinetically unfavorable. In addition, the catalyst would need to selectively distinguish between the two similar olefins present in substrate I for the initial borocupration. Herein, we report the successful implementation of our proposed strategy. The key to our success lay in the use of a copper catalyst supported by a bulky, electron-rich monodentate biarylphosphine ligand (CyJohnPhos, L1) developed in the Buchwald lab.[8]
Scheme 1.
Copper-Catalyzed Borylation/Cyanation/Cope Rearrangement: A Strategy for the Regio- and Stereospecific 1,3-Allyl Transposition.
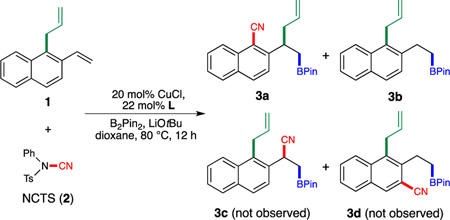
Using NCTS (2) as the electrophilic cyanating reagent,[9] we examined the proposed tandem borylation/cyanation/allyl transfer by evaluating a series of copper catalysts that were previously used to promote styrene borocupration.[10] We found that the catalyst derived from CyJohnPhos (L1) facilitated the desired transformation, providing the cyanation/allyl migration product 3a in 62% yield (entry 1). The structure of 3a was further confirmed by 2D NMR spectroscopy (1H-1H COSY). Hydroboration product 3b lacking the cyano group represented the only other product observed. Notably, neither the benzylic cyanation product 3c nor the C–H cyanation product 3d was formed as indicated by 1H NMR spectroscopic analysis. Interestingly, replacement of L1 with tricyclohexylphosphine (L2) resulted in a catalyst that was only capable of promoting the undesired hydroboration (entry 2). Further evaluation of ligand effects revealed that commonly used bidentate phosphine ligands were much less effective (entry 3–6). Finally, a preformed cationic copper(I)-phosphine complex[11] [(L1)Cu][OTf] (4) was identified as an excellent precatalyst for facilitating this reaction (entry 7).
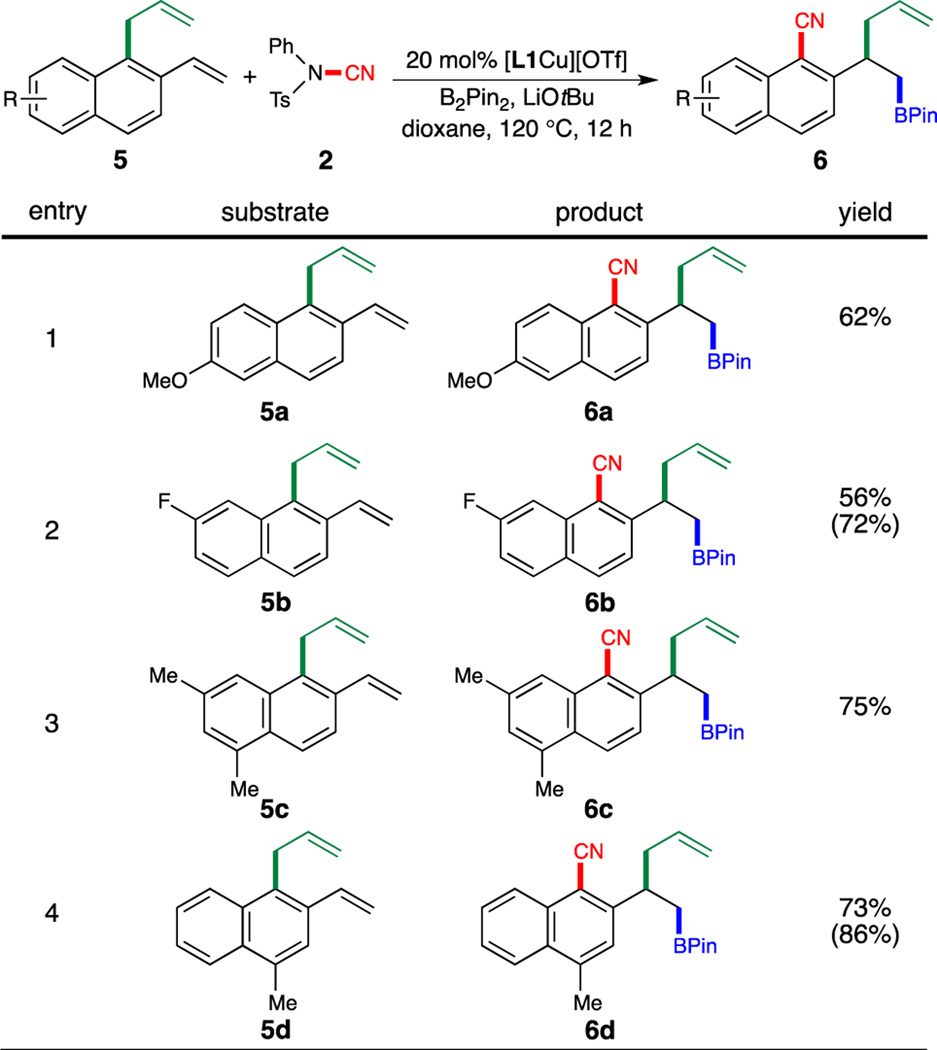
We next set out to explore the substrate scope of this borylation/cyanation/allyl migration reaction (Scheme 2 and 3). A variety of substituted 1-allyl-2-vinylnaphthalenes bearing electron-donating or electron-withdrawing functional groups were found to be excellent substrates (Scheme 2, 6a–6e).
Scheme 2.
Substrate Scope of Arenes. Reaction Conditions: 5 (0.20 mmol), 2 (0.24 mmol), 4 (0.040 mmol), B2Pin2 (0.22 mmol), LiOtBu (0.24 mmol), dioxane (0.50 mL), 120 °C, 12 h. Isolated yields were reported. Yields in parentheses were determined by 1H NMR analysis of the crude reaction mixture using 1,1,2,2-tetrachloroethane as an internal standard. Isolated yields were 5–20% lower than 1H NMR yields due to product decomposition on silica gel.
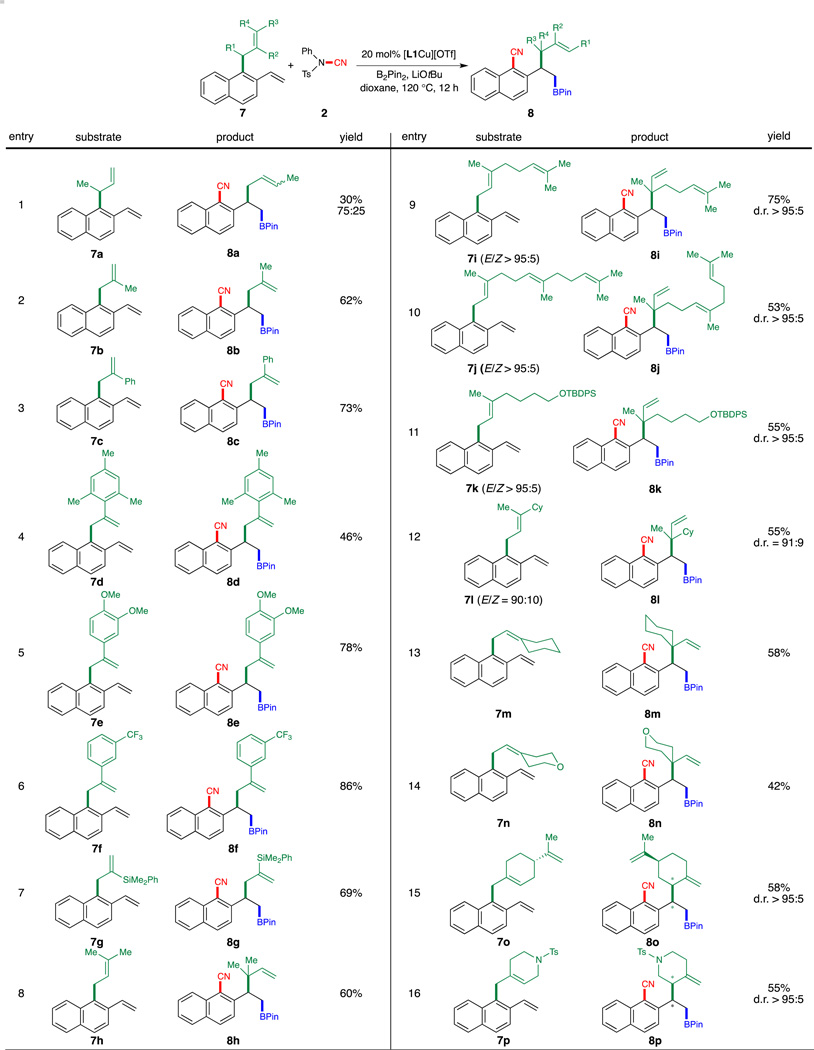
Scheme 3.
Substrate Scope of the Migrating Allyl Groups. Reaction Conditions: 7 (0.20 mmol), 2 (0.24 mmol), 4 (0.040 mmol), B2Pin2 (0.22 mmol), LiOtBu (0.24 mmol), dioxane (0.50 mL), 120 °C, 12 h. Isolated yields were reported. Isolated yields were usually 5–15% lower than 1H NMR yields due to product decomposition on silica gel.
In addition, this process showed a broad substrate scope with respect to the allyl component (Scheme 3). Arenes possessing 2-substituted allyl groups (7b–7g) provided corresponding products in good to excellent yields. Owing to the increased steric hindrance proximal to the cyanated carbon, 7a furnished the cyanation/allyl transfer products in moderate yield as a 75:25 mixture of olefin stereoisomers. Furthermore, 3,3-disubstituted allyl components were also compatible with the current reaction (7h–7l). In the case of 3,3-unsymmetrically disubstituted allyl substrates, the stereochemical information of the olefin geometry was fully transferred to the product in the form of relative configuration of the newly formed vicinal stereocenters (7i–7l). Cyclic variant 7m and its heterocyclic analogue 7n also represented viable substrates for this reaction. Finally, allylvinylarenes bearing an endocyclic C=C double bond (7o and 7p) could be successfully transformed into the cyanation/allyl migration product with excellent diastereoselectivity.
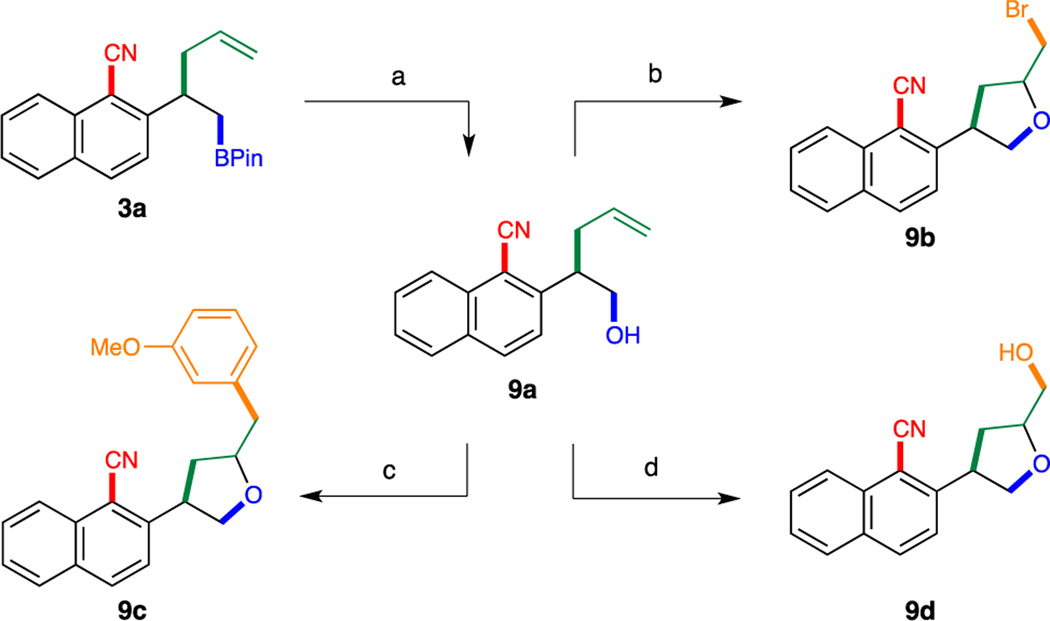
To further demonstrate the synthetic utility of this transformation, several derivatization reactions were carried out (Scheme 4). Oxidation of cyanoboronate 3a under mild conditions afforded alcohol 9a in 90% yield.[12] By taking advantage of the olefin and the alcohol functional groups present in 9a, an array of olefin difunctionalization reactions, including bromoetherification[13] (9b), palladium-catalyzed oxyarylation[14] (9c) and dioxygenation[15] (9d), were successfully accomplished to furnish various complex 2,4-disubstituted tetrahydrofurans in excellent yields.
Scheme 4.
Derivatization of Borylation/Cyanation/Allyl Transfer Products. Conditions: a. NaBO3·4H2O, THF/H2O, RT, 2 h (90%). b. NBS, NaHCO3, CH2Cl2/H2O, RT, 12 h (97%, d.r. = 78:22). c. 2.5 mol % [(allyl)PdCl]2,10 mol % SPhos, 3-bromoanisole, NaOtBu, toluene, 110 °C, 12 h (71%, d.r. = 62:38). d. m-CPBA, CH2Cl2, RT, 48 h (96%, d.r. = 50:50). Isolated yields were reported.
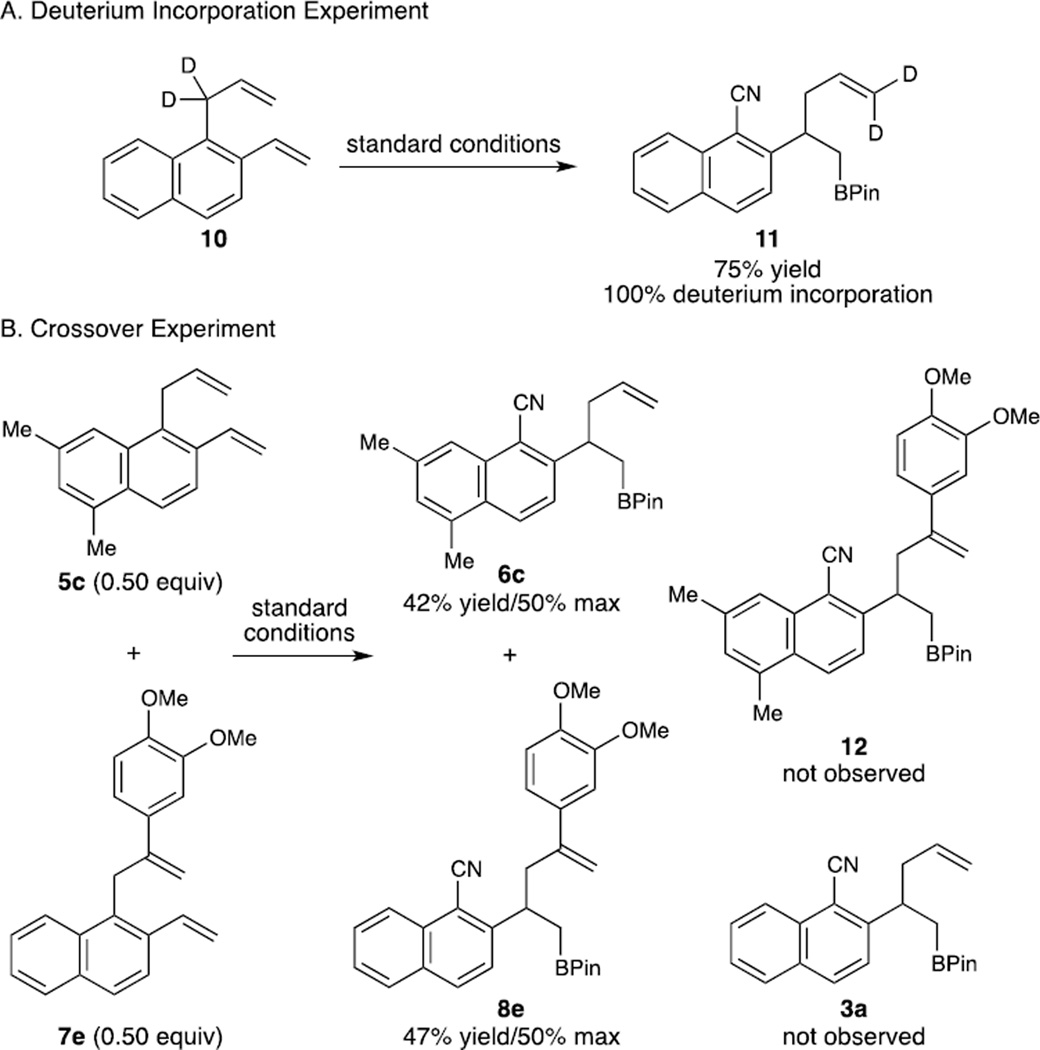
In an effort to gain insight into the mechanism of the reaction described above, we prepared deuterated substrate 10 and subjected it to the standard reaction conditions (Scheme 5). It was found that both deuterium atoms were fully incorporated into product 11 at the terminal position of the olefin. This finding suggests that an overall 1,3-transposition of the allyl moiety has taken place. Furthermore, having confirmed that 5c and 7e reacted at similar rates, we conducted a crossover experiment using these two substrates. Careful examination of the 1H NMR spectrum revealed that neither of the crossover product 12 and 3a was present in the crude reaction mixture, whereas the yield of the unimolecular allyl migration products 6c and 8e remained essentially unaffected. This observation is consistent with an intramolecular 1,3-allyl group migration mechanism.
Scheme 5.
Mechanistic Studies.
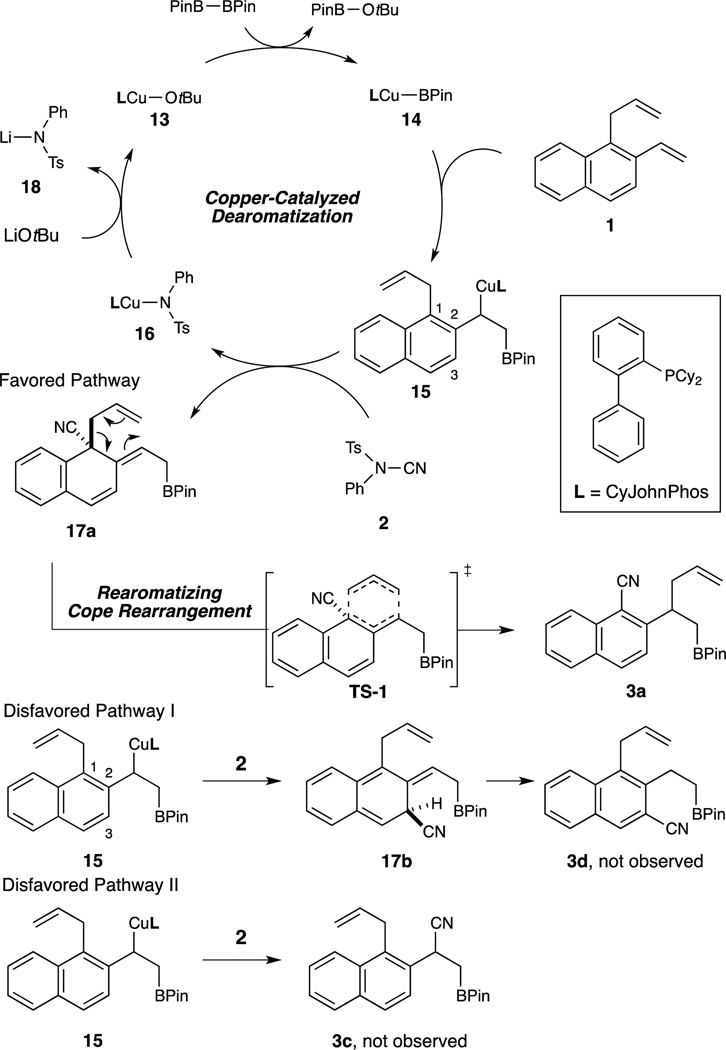
Based on these findings, a plausible reaction mechanism is shown in Scheme 6. Transmetalation of the monodentate phosphine ligated copper catalyst 13 with the diboron reagent provides the copper-boryl complex 14, which undergoes borocupration with 1 to provide the benzylcopper species 15. Electrophilic cyanation of 15 affords the dearomatized product 17a, eventually leading to the formation of 3a through the rearomatizing Cope rearrangement. Complete regio- and stereospecificity is ensured by the highly organized chair-like transition state of this [3,3]-sigmatropic rearrangement. We believe the electron-rich CyJohnPhos (L1) greatly enhances the nucleophilicity of 15 and is thus critical in promoting this challenging quaternary carbon-forming dearomatization process. Cyanation at the C3 position (17b) would disrupt the aromaticity of both benzene rings and is thus thermodynamically disfavored. We are performing computational investigations to gain further insight into the preferred C1 cyanation over the benzylic cyanation as well as the nature of the transition state for this rearomatizing Cope rearrangement.
Scheme 6.
Mechanistic Rationale.
In summary, we have developed a novel catalytic borylation/ortho-cyanation/Cope rearrangement sequence. This cascade process features the overall regio- and stereospecific 1,3-allyl transposition, which proceeds through a rearomatizing [3,3]-sigmatropic rearrangement triggered by the copper-catalyzed electrophilic cyanative dearomatization. This protocol provides an effective means to access a broad range of synthetically useful building blocks that can be easily transformed into a variety of complex molecular scaffolds. The results presented herein may serve as a platform for the development of an enantioselective allyl transposition reaction as well as other rearrangement processes initiated by dearomatization.
Supplementary Material
Table 1.
Optimization of Reaction Conditionsa.
 | |||
|---|---|---|---|
| entry | L | yield of 3a | yield of 3b |
| 1 | L1 | 62% | 16% |
| 2[b] | L2 | <5% | 66% |
| 3 | L3 | <5% | <5% |
| 4 | L4 | <5% | 51% |
| 5 | L5 | 18% | 10% |
| 6 | L6 | 6% | 19% |
| 7[c] | L1 | 74% | 13% |
Reaction conditions: 1 (0.10 mmol), 2 (0.12 mmol), CuCl (0.020 mmol), L (0.022 mmol), B2Pin2 (0.11 mmol), LiOtBu (0.12 mmol), dioxane (0.25 mL), 80 °C, 12 h. Yields were determined by 1H NMR analysis of the crude reaction mixture using 1,1,2,2-tetrachloroethane as an internal standard.
40 mol% L.
[(L1)Cu][OTf] (4) (20 mol%) was used in lieu of CuCl and L1, 120 °C.
Acknowledgments
We are grateful to Prof. Stephen L. Buchwald (MIT) for his support and encouragement. We thank Dr. Yi-Ming Wang (MIT) for helpful discussions. This work is supported by the National Institutes of Health (GM46059 for S.L.B.).
References
- 1.Cope AC, Hardy EM. J Am. Chem. Soc. 1940;62:441. [Google Scholar]
- 2.Selected reviews on Cope rearrangement: Lutz RP. Chem. Rev. 1984;84:205. Paquette LA. Angew. Chem. Int. Ed. 1990;29:609. Paquette LA. Tetrahedron. 1997;53:13971. Nubbemeyer U. Synthesis. 2003;7:961. Jones AC, May J, Sarpong R, Stoltz BM. Angew. Chem. Int. Ed. 2014;53:2. doi: 10.1002/anie.201302572.
- 3.a) Yang Y, Buchwald SL. Angew. Chem. Int. Ed. 2014;53:8677. doi: 10.1002/anie.201402449. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yang Y, Liu P. ACS Catal. 2015;5:2944. [Google Scholar]
- 4.For a recent review on the reactivity of transition-metal benzyl complexes: Trost BM, Czabaniuk LC. Angew. Chem. Int. Ed. 2014;53:2826. doi: 10.1002/anie.201305972.
- 5.Zhao W, Montgomery J. Angew. Chem. Int. Ed. 2015 [Google Scholar]
- 6.a) Grigg RD, van Hoveln R, Schomaker JM. J Am. Chem. Soc. 2012;134:16131. doi: 10.1021/ja306446m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Van Hoveln RJ, Schmid SC, Schomaker JM. Org. Biomol. Chem. 2014;12:7655. doi: 10.1039/c4ob01294a. [DOI] [PubMed] [Google Scholar]; c) Van Hoveln RJ, Schmid SC, Tretbar M, Buttke CT, Schomaker JM. Chem. Sci. 2014;5:4763. doi: 10.1039/C4SC02040E. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Van Hoveln R, Hudson BM, Wedler HB, Bates DM, Le Gros G, Tantillo DJ, Schomaker JM. J Am. Chem. Soc. 2015;137:5346. doi: 10.1021/ja511236d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selected reviews on transition-metal-catalyzed C–C bond activation: Crabtree RH. Chem. Rev. 1985;85:245. Jones WD. Nature. 1993;364:676. Rybtchinski B, Milstein D. Angew. Chem. Int. Ed. 1999;111:918. doi: 10.1002/(SICI)1521-3773(19990401)38:7<870::AID-ANIE870>3.0.CO;2-3. van der Boom ME, Milstein D. Chem. Rev. 2003;103:1759. doi: 10.1021/cr960118r. Murakami M, Ito Y. Top. Organomet. Chem. 1999;3:97. Murakami M, Matsuda T. Chem. Comm. 2011;47:1100. doi: 10.1039/c0cc02566f. Jun C-H. Chem. Soc. Rev. 2004;33:610. doi: 10.1039/b308864m. Jun C-H, Park JW. Top. Organomet. Chem. 2007;24:117. Seiser T, Saget T, Tran DN, Cramer N. Angew. Chem. Int. Ed. 2011;50:7740. doi: 10.1002/anie.201101053. Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117. doi: 10.1021/cr050988l. Flores-Gaspar A, Martin R. Synthesis. 2013;45:563. Dermenci A, Cow JW, Dong G. Org. Chem. Front. 2014;1:567. doi: 10.1039/c4qo00053f. Xu T, Dermenci A, Dong G. Top. Curr. Chem. 2014;346:233. doi: 10.1007/128_2014_545. Kondo T, Mitsudo T. Chem. Lett. 2005;34:1462.
- 8.Wolfe JP, Buchwald SL. Angew. Chem. Int. Ed. 1999;38:2413. doi: 10.1002/(sici)1521-3773(19990816)38:16<2413::aid-anie2413>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.NCTS (2) is a safe and bench stable crystalline reagent that can be easily prepared from tosyl chloride and phenylurea. For selected applications of NCTS in electrophilic cyanation: Anbarasan P, Neumann H, Beller M. Angew. Chem. Int. Ed. 2011;50:519. doi: 10.1002/anie.201006044. Anbarasan P, Neumann H, Beller M. Chem. Eur. J. 2011;17:4271. doi: 10.1002/chem.201003388. Yang Y, Zhang Y, Wang J. Org. Lett. 2011;13:5068. doi: 10.1021/ol202335p. Gong T-J, Xiao B, Cheng W-M, Su W, Xu J, Liu Z-J, Liu L, Fu Y. J Am. Chem. Soc. 2013;135:10630. doi: 10.1021/ja405742y. Chaitanya M, Yadagiri D, Anbarasan P. Org. Lett. 2013;15:4960. doi: 10.1021/ol402201c. Liu W, Ackermann L. Chem. Comm. 2014;50:1878. doi: 10.1039/c3cc49502g. Yu D-G, Gensch T, de Azambuja F, Vasquez-Cespedes S, Glorius F. J Am. Chem. Soc. 2014;136:17722. doi: 10.1021/ja511011m. Li J, Ackermann L. Angew. Chem. Int. Ed. 2015;54:3635. doi: 10.1002/anie.201409247.
- 10.Examples of borocupration of styrenes: Laitar DS, Tsui EY, Sadighi JP. Organometallics. 2006;25:2405. Lillo V, Fructos MR, Ramírez J, Braga AAC, Maseras F, Díaz-Requejo MM, Pérez PJ, Fernández E. Chem. Eur. J. 2007;13:2614. doi: 10.1002/chem.200601146. Lee Y, Hoveyda AH. J Am. Chem. Soc. 2009;131:3160. doi: 10.1021/ja809382c. Zhong C, Kunii S, Kosaka Y, Sawamura M, Ito H. J Am. Chem. Soc. 2010;132:11440. doi: 10.1021/ja103783p. Ito H, Toyoda T, Sawamura M. J Am. Chem. Soc. 2010;132:5990. doi: 10.1021/ja101793a. Corberán R, Mszar NW, Hoveyda AH. Angew. Chem., Int. Ed. 2011;50:7079. doi: 10.1002/anie.201102398. Matsuda N, Hirano K, Satoh T, Miura M. J Am. Chem. Soc. 2013;135:4934. doi: 10.1021/ja4007645. Samba K, Nakao Y. J Am. Chem. Soc. 2014;136:7567. doi: 10.1021/ja5029556. Smith KB, Logan KM, Brown MK. Chem. –Eur. J. 2014;20:12032. doi: 10.1002/chem.201404310. Logan KM, Smith KB, Brown MK. Angew. Chem., Int. Ed. 2015;54:5228. doi: 10.1002/anie.201500396.
- 11.Pérez-Galán P, Delpont N, Herrero-Gómez E, Maseras F, Echavarren AM. Chem. Eur. J. 2010;16:5324. doi: 10.1002/chem.200903507. [DOI] [PubMed] [Google Scholar]
- 12.Kubota K, Yamamoto E, Ito H. J Am. Chem. Soc. 2013;135:2635. doi: 10.1021/ja3104582. [DOI] [PubMed] [Google Scholar]
- 13.Jurberg ID, Odabachian Y, Gagosz F. J Am. Chem. Soc. 2010;132:3543. doi: 10.1021/ja9100134. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe JP, Rossi MA. J Am. Chem. Soc. 2004;126:1620. doi: 10.1021/ja0394838. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Thai K, Gravel M. Org. Lett. 2009;11:891. doi: 10.1021/ol8029005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.