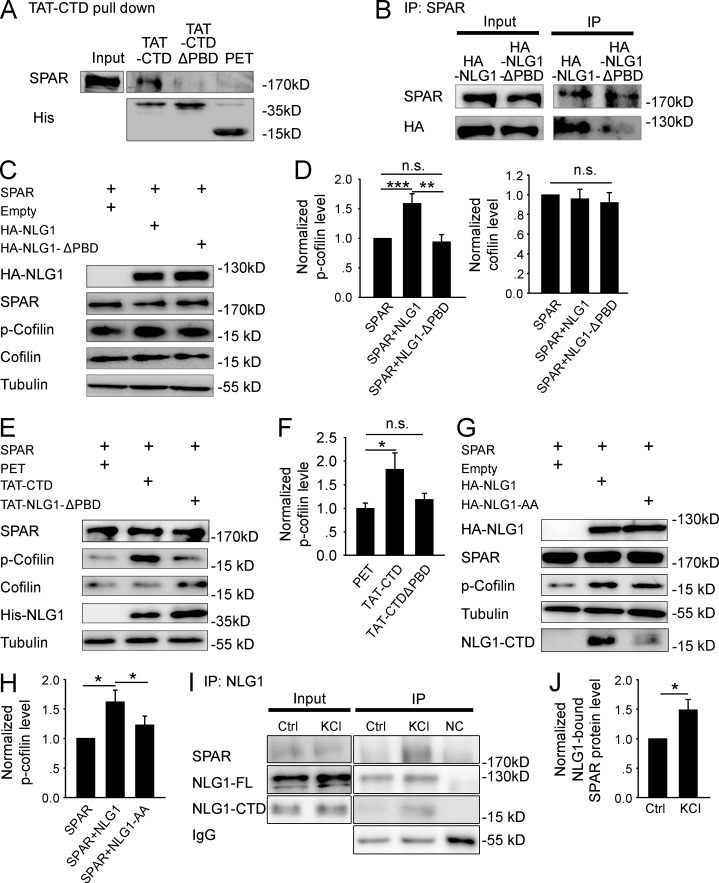
Figure 4.
NLG1 CTD regulates cofilin phosphorylation through interaction with SPAR. (A) Western blots of pull-down experiments of brain lysate showing that TAT-CTD, but not TAT-CTDΔPBD or PET, could pull down SPAR. (B) Western blots of anti-SPAR immunoprecipitates using protein lysate of HEK293 cells cotransfected with myc-SPAR plus HA-NLG1 or HA-NLG1ΔPBD showing that HA-NLG1, but not HA-NLG1-ΔPBD, was coimmunoprecipitated with SPAR. (C) Western blots of protein lysate of HEK293 cells cotransfected with myc-SPAR and HA-NLG1 or HA-NLG1ΔPBD. (D) Summary graphs of C showing significantly increased p-cofilin, but not total cofilin, in HEK293 cells cotransfected with myc-SPAR and HA-NLG1, compared with those transfected with myc-SPAR and HA-NLG1ΔPBD or with myc-SPAR alone. n.s., not significant. (E) Western blots of protein lysate of myc-SPAR–transfected HEK293 cells treated with the recombinant proteins. (F) Summary graph of E showing significantly increased p-cofilin in TAT-CTD, compared with TAT-CTDΔPBD– or PET-treated HEK293 cells pretransfected with myc-SPAR. n.s., not significant. (G) Western blots of protein lysate of HEK293 cells cotransfected with myc-SPAR plus HA-NLG1 or HA-NLG1-AA. (H) Summary graph of G showing significantly higher p-cofilin in myc-SPAR and HA-NLG1–cotransfected HEK293 cells compared with myc-SPAR and HA-NLG1-AA cotransfections. (I) Western blots of anti-NLG1 immunoprecipitates of hippocampal slices with or without KCl treatment. NC, negative IP control with rabbit IgG. (J) Summary graph of I showing significantly increased NLG1-bound SPAR after KCl treatment. Ctrl, control. *, P < 0.05; **, P < 0.01; ***, P < 0.001.