Dejana’s extensive tenure covers the depths of endothelial cells.
Dejana’s extensive tenure covers the depths of endothelial cells.
Elisabetta Dejana’s last name, which is uncommon in Italy, throws people off. The “j,” which is rarely found in Italian words, is pronounced like a “y” in English.

Elisabetta Dejana
PHOTO COURTESY OF ELISABETTA DEJANA
She likes to give the backstory. On the island of Sardinia, where her father grew up, there were caves where women left illegitimate babies to be raised by nuns. The caves were called the houses “of the witches,” or “de janas,” in the Sardinian dialect. “So probably my great-great-great-grandfather was an abandoned baby,” she says.
With this origin story, Dejana reveals a sketch of herself—someone who abandoned the traditional roles for an Italian woman to pursue a life-long study of blood vessels. After completing her doctorate at Mario Negri Institute for Pharmacological Research in Milan, Dejana made the first of many international moves to do postdoctoral work in the early 1980s at McMaster University in Hamilton, Ontario, Canada. Now she splits her time between being the chief of the vascular biology program at the FIRC Institute for Molecular Oncology in Milan and leading a group at Uppsala University in Sweden.
Her group discovered vascular endothelial (VE)-cadherin (1), the first tissue-specific cadherin identified. More recently, her labs have focused on the role of endothelial cells in angiogenesis (2), in blood–brain barrier formation (3), and in the devastating disorder, cerebral cavernous malformations (4, 5).
“Endothelial cells change their functions to adapt to the physiology of different organs.”
Dejana recently spoke with JCB from her Milan office about the multifunctional endothelium and getting back to the land.
TAKE A SECOND LOOK
Why have endothelial cells, which line blood vessels, held your fascination for so long?
For many years, endothelial cells were considered a passive, structural layer of cells. But, when we started to work with these cells, we realized that they produce proteins and molecules to prevent thrombosis, modulate blood flow by contracting or dilating the vessel, and regulate vascular permeability.
So they are extremely interactive cells. Working on endothelial cells also crosses into lots of fields such as tumorigenesis, inflammation, and atherosclerosis.
How did you end up working on cadherins?
When I moved back to Italy, I started to develop a technique of isolating and culturing umbilical cord endothelial cells. From these cultured cells, I became interested in adhesion proteins. We tried to identify the major integrins in endothelial cells to see their roles in cell migration, growth, and repair of vascular injury.
We decided to develop our own monoclonal antibodies and while we were screening them, we detected some that decorated the cell–cell junctions. I caught people throwing these antibodies away! I was quick to stop them and say this was much more interesting than integrins, because no one knew anything about the endothelial cell–cell junctions. The antibody staining was also beautiful. The antibodies decorated these borders so clearly that it was easy to think they could be scientifically relevant.
One of the proteins we discovered was VE-cadherin, a cadherin specific for endothelial cells. This also introduced the concept that each cell type could express specific cadherins, which is important for the assembly of tissues because they promote homotypic cell–cell adhesion.
ALTERING BRAIN MICROVASCULATURE
How did you turn from angiogenesis to the blood–brain barrier (BBB)?
We were studying VE-cadherin signal transduction. VE-cadherin is physically linked to β-catenin and, when the VE-cadherin complex is dismantled, there is an increase of β-catenin in the nucleus where it acts as a transcriptional modulator. Nuclear β-catenin levels were very high during development of the brain microvasculature and we thought this could be responsible for the specialization of the brain’s vascular system.
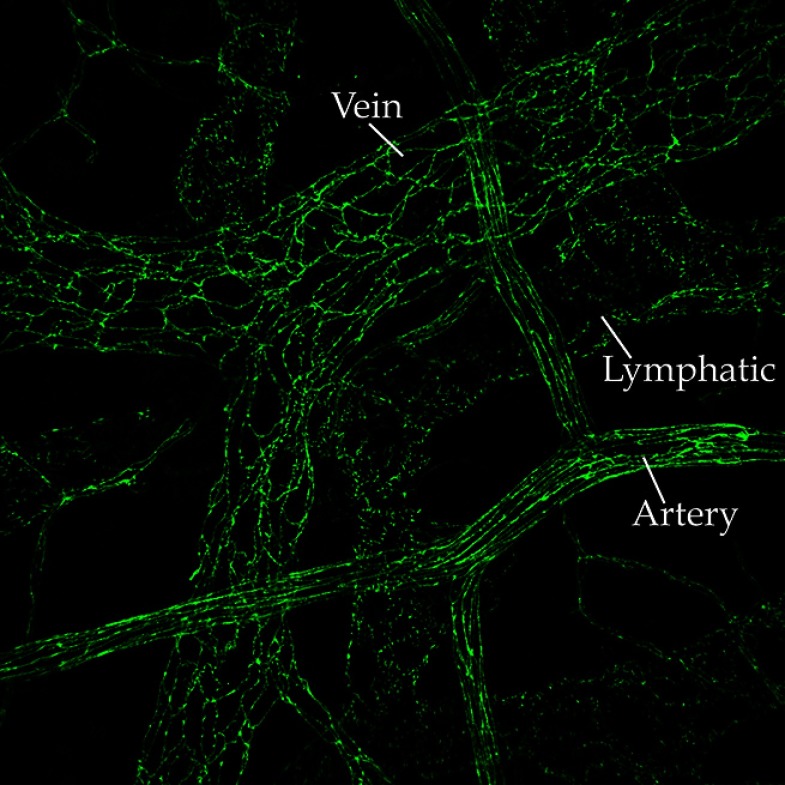
VE-cadherin shows a zipper-like distribution at endothelial cell–cell contacts in mouse ileum.
IMAGE COURTESY OF ELISABETTA DEJANA
Endothelial cells change their functions to adapt to the physiology of different organs. Endothelial cells in the brain microvasculature should have tight control of permeability because they protect the central nervous system from circulating toxic agents and inflammatory cells.
So we became interested in the BBB and the role of Wnt signaling, which stabilizes β-catenin. The possibility of opening the BBB would have important therapeutic implications—including getting chemotherapy to brain tumors or improving the access of drugs for neurological disorders.
What did you find out about the BBB?
Using a reporter mouse, we saw that Wnt signaling in the brain was very high, and it followed the time course of BBB development during the late stages of embryonic development and shortly after birth.
When we introduced a mutation that stabilized β-catenin, BBB differentiation was accelerated. And, when we abrogated β-catenin expression or signaling, BBB development was impaired and the organization of endothelial cell–cell junctions was strongly altered.
Your group has been investigating cerebral cavernous malformations, or CCM…
In CCM, endothelial cell–cell junctions are disorganized and the cells start to grow in an uncontrolled way. The vessels form cavernomas, which are shaped like big raspberries, with multiple lumens. These patients tend to bleed in their brain, which causes major problems—headaches, epileptic crises, and even paralysis or psychosis—and some die from a hemorrhagic stroke.
The familial disease is characterized by mutations in one of three genes, CCM1, CCM2, and CCM3, which encode three proteins that form a complex. It can also be a sporadic disease—the total prevalence of CCM is 1 in 200.
How do you investigate this disorder at the cellular level?
We followed the kinetics of this phenomenon in mice with a null mutation of any one of the three CCM genes. These mice develop malformations similar to those in humans. The first sign is an enlargement of the vascular lumen and the endothelial cells lose the correct organization and architecture of their cell–cell junctions. This is followed by β-catenin signaling to the nucleus. Then, β-catenin signaling declines and there is an increase in TGF-β signaling.
We can use these mouse models to screen for pharmacological agents that reduce or stabilize these malformations. We are currently trying to take one particular drug therapy forward. To tell you the truth, my aspiration is, at the end of all these studies, to be able to help patients. Everyday I receive e-mails from CCM patients because of our work. CCM patients come to the institute and it helps my lab realize how important it is to try to alleviate their suffering.
WORK-LEISURE TRAVEL
You’ve worked in many places around the globe doing research sabbaticals. Do you have a favorite research environment?
I have difficulty picking only one. When I worked in Canada, I was very young and everything was a discovery. I had the privilege to be a postdoc with Fraser Mustard who transferred an interest in translational research to me.
“My aspiration is, at the end of all these studies, to be able to help patients.”
Dejana’s lab celebrates the acceptance of a JCB paper.
PHOTO COURTESY OF ELISABETTA DEJANA
Harvard Medical School in Boston, where I worked as a young scientist was also a fantastic experience. I had the chance to collaborate with Michael Gimbrone who introduced me to the cell biology of endothelial cells and I was really amazed by how the whole Department of Pathology, directed by Ramzi Cotran, was scientifically rich and stimulating.
Every place gave me a different perspective and this is very important in our work. Italy is a relatively small country, and so I needed to travel to find other groups doing similar things. I’m trying to push my lab workers to do more networking—young people should go out of their country.
Do you have other advice for young scientists?
I am particularly interested in helping women scientists, because I think that they are disadvantaged for many reasons. So my first simple suggestion is, you need to be persistent. Second, you need to be passionate. And the third important part is to choose a good companion.
It seems funny to say, but having a research career is really difficult. If you want to do it at a good level, you do need support. I’ve seen so many brilliant women leaving science because they couldn’t attend to their family life if their partner didn’t help them. This is unjust.
It doesn’t seem as if you have any plans to retire soon. What do you do when you are not in the lab?
As a full professor at the University of Milan, retirement is at 70, so I still have some time! [Laughs]
One passion I have is the mountains. I love to climb up them and then ski down in fresh snow. There are fantastic mountains close to Milan—Mont Blanc and Monte Rosa.
My second passion is a farm that we just bought in southern Italy. We have a lot of olive trees and we produce olive oil for friends. It’s incredibly nice to go back to the country, to the land.
References
- 1.Lampugnani M.G., et al. . 1995. J. Cell Biol. 129:203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P., et al. . 1999. Cell. 98:147–157. [DOI] [PubMed] [Google Scholar]
- 3.Liebner S., et al. . 2008. J. Cell Biol. 183:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maddaluno L., et al. . 2013. Nature. 498:492–496. [DOI] [PubMed] [Google Scholar]
- 5.Bravi L., et al. . 2015. Proc. Natl. Acad. Sci. USA. 112:8421–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]