Figure 3.
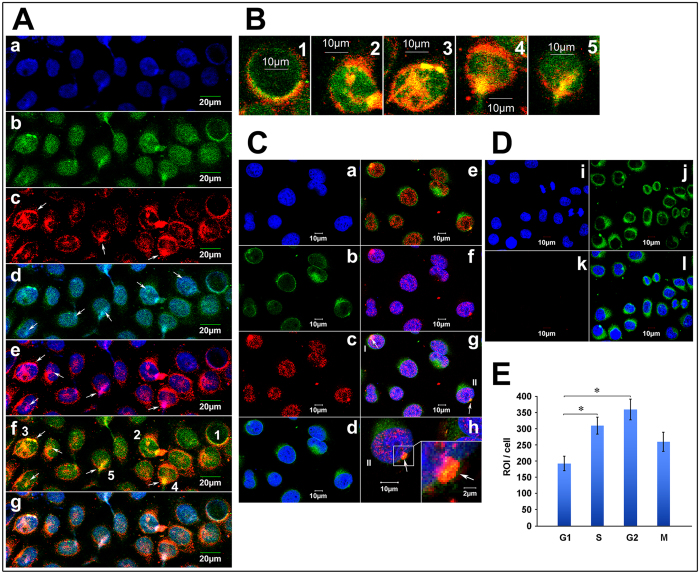
Co-localization of Cx43, AKAP95 and DNA in A549 cells (A) Co-localization of Cx43 and AKAP95 in the nucleus. (Aa) Blue fluorescence represents DAPI-labeled nuclei; (Ab) green fluorescence represents FITC-labeled AKAP95; (Ac) red fluorescence represents TXRD-labeled Cx43; (Ad) merged image of Aa and Ab; (Ae) merged image of Aa and Ac; (Af) merged image of Ab and Ac; and (Ag) merged image of Aa, Ab, and Ac; (B) Rows 1–5 are the enlarged images of cells 1–5 in Af. (magnification 200 × for A; 200 × 4 for B). (C) Proximity ligation assay and immunofluorescence detected by Laser scanning confocal microscope (200 × ). Labeling with DAPI is shown in (Ca), and lamin B1 is shown in (Cb). In (Cc), the combination of AKAP95 and Cx43 was labeled with TXRD. Results showed interaction between AKAP95 and Cx43 (red). Both proteins were localized in the nucleus. Panel (Cd) is a merged image of (Ca) and (Cb). Panel (Ce) is a merged image of (Cb) and (Cc). Panel (Cf) is a merged image of (Ca) and (Cc). In panel (Cg) (merged image of Ca, Cb and Cc), cells highlighted as I and II point to the combination of AKAP95 and Cx43 crossing the nuclear membrane. Cells II are enlarged in (Ch) (200 × 4). At the lower right corner (200 × 4 × 4), the junction between complex and membrane was demonstrated by yellow fluorescence(arrow), suggesting movement into the nucleus via the nuclear membrane. (D) is the negative control to C. (Di) is DAPI, (Dj) is Lamin B1, (Dk) is Cx43 primary antibody replace by PBS as a negative control in PLA assays; red fluorescence was not detected. Panel (Dl) is a merged image of (Di), (Dj) and (Dk). (E) We detected the quantitative changes of AKAP95/Cx43 complexes in G1, S, G2, M phases using Olympus software in PLA assays. The number of the detected cells was 37 in G1 phase, 30 in S phase, 13 in G2 phase and 10 in M phase. In addition, there were statistically significant differences of the amount of AKAP95/Cx43 complexes between G1 and S phase, G1 and G2 phase respectively.