Figure 2.
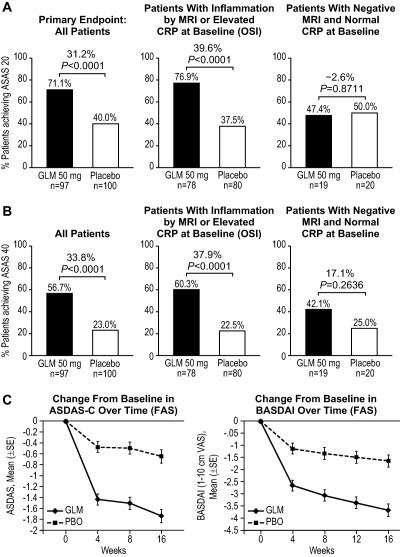
A and B, Percentages of patients in the golimumab (GLM) and placebo (PBO) groups achieving 20% improvement according to the Assessment of SpondyloArthritis international Society criteria (ASAS20) (A) and ASAS40 criteria (B) at week 16, in the full analysis set (FAS) and according to magnetic resonance imaging (MRI) status (positive or negative for the presence of sacroiliitis) and C‐reactive protein (CRP) status (objective signs of inflammation [OSI] population). Differences were derived using the stratified method described by Miettinen and Nurminen (30). C, Change from baseline in the Ankylosing Spondylitis Disease Activity Score using the CRP level (ASDAS‐C) and change from baseline in the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) response over time.
