Abstract
Acquired generalized lipodystrophy (AGL) is a rare disease thought to result from autoimmune destruction of adipose tissue. Peripheral T-cell lymphoma (PTCL) has been reported in 2 AGL patients. We report five additional cases of lymphoma in AGL, and analyze the role of underlying autoimmunity and recombinant human leptin (metreleptin) replacement in lymphoma development.
Three patients developed lymphoma during metreleptin treatment (two PTCL and one ALK-positive anaplastic large cell lymphoma, and two developed lymphomas (mycosis fungoides and Burkitt lymphoma) without metreleptin.
AGL is associated with high risk for lymphoma, especially PTCL. Autoimmunity likely contributes to this risk. Lymphoma developed with or without metreleptin, suggesting metreleptin does not directly cause lymphoma development; a theoretical role of metreleptin in lymphoma progression remains possible. For most patients with AGL and severe metabolic complications, the proven benefits of metreleptin on metabolic disease will likely outweigh theoretical risks of metreleptin in lymphoma development or progression.
Keywords: T-cell lymphoma, Acquired generalized lipodystrophy, Metreleptin, Leptin, Mycosis fungoides
INTRODUCTION
The lipodystrophies are a heterogeneous group of rare diseases that have in common selective deficiency of adipose tissue1. There may be near-total deficiency of subcutaneous fat, termed generalized lipodystrophy, or selective deficiency of fat in certain subcutaneous depots, with preservation or even hypertrophy of other fat depots, termed partial lipodystrophy. Both generalized and partial lipodystrophies may be inherited or acquired, leading to four broad categories of lipodystrophy: congenital generalized lipodystrophy, familial partial lipodystrophy, acquired generalized lipodystrophy (AGL), and acquired partial lipodystrophy. Genes implicated in inherited lipodystrophies are involved in lipid droplet formation, or adipocyte differentiation or survival. Acquired lipodystrophies are thought to result from autoimmune destruction of fat cells, although the target(s) for the autoimmune attack are not known.
The paucity of fat in lipodystrophy results in low levels of fat-derived hormones, including leptin2. Leptin deficiency, in turn, leads to hyperphagia, and excess calories are stored ectopically in the liver and muscle. The combination of ectopic lipid deposition and leptin deficiency leads to severe metabolic complications at an early age. These complications resemble a severe variant of the obesity-associated metabolic syndrome, and include diabetes with extreme insulin resistance, hypertriglyceridemia, non-alcoholic steatohepatitis, and polycystic ovarian syndrome. Leptin replacement in patients with lipodystrophy improves hyperphagia and metabolic complications of lipodystrophy3–8, and a recombinant analog of human leptin (metreleptin) was recently approved by the United States Food and Drug Administration (FDA) for treatment of complications of leptin deficiency in patients with generalized lipodystrophies, including both genetic and acquired forms. However, the FDA included two warnings in the package insert, for T-cell lymphoma in patients with AGL, and for the development of anti-metreleptin antibodies with in vitro neutralizing activity, both of which were observed during the single-arm uncontrolled studies of metreleptin at the National Institutes of Health (NIH) that were pivotal for the FDA approval.
In the current publication, we describe the three cases of lymphoma that occurred during metreleptin treatment in the NIH studies, as well as two additional cases that occurred in patients with AGL without metreleptin treatment. We further assess potential contributors to the development of lymphoma in AGL, including possible roles for autoimmunity and metreleptin treatment.
METHODS
Cases 1–3 participated in a prospective, one-arm, open-label study of metreleptin at the NIH (NCT00025883). The study was approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases. Written informed consent was obtained from patients or their legal guardians. Assent was obtained from minors. Metreleptin was self-administered subcutaneously once or twice daily at doses of 0.06 to 0.24 mg/kg/day, with adjustment based on metabolic control. In cases 1–3, pathologic specimens were examined by a single pathologist at the NIH (ESJ). A literature review was conducted to identify published cases of lymphoma in patients with acquired generalized lipodystrophy.
Role of the funding source: The authors are solely responsible for the study design, the collection, analysis, and interpretation of data, the writing of the manuscript, and the decision to submit for publication.
RESULTS
All cases reported here, as well as two previously published cases9,10, are summarized in Table I.
TABLE I.
Clinical characteristics of patients with acquired generalized lipodystrophy and lymphoma.
| Case | Age at lipo-dystrophy development (years) | Age at lymphoma diagnosis (years) | Lymphoma type | Autoimmune markers | Other clinical features | Metreleptin treated at lymphoma diagnosis? |
|---|---|---|---|---|---|---|
| 1 | 63 | 68 | Peripheral T-cell lymphoma | Negative ANA, ENA, TG, TPO; normal C3/C4/TC. | Neutropenia | yes |
| 2 | ~49 | 59 | Peripheral T-cell lymphoma | Low C4 (5 mg/dL, normal 13–38), high TC (154 CAE units, normal 55–145). Negative ANA, AMA, cardiolipin, ENA, GAD, anti-islet, TG, TPO; normal C3, IgA/IgG/IgM. | Neutropenia, breast cancer | yes |
| 3 | 1 | 13 | ALK+ anaplastic large cell lymphoma | Negative AMA, ANA, ANCA, cardiolipin, ENA, GAD, TG, TPO, RF; normal C3/C4/TC, IgA/IgG/IgM | None | yes |
| 4 | 60 | 39 | Mycosis fungoides | Not performed | Adeno-carcinoma | no |
| 5 | ~10 | 16 | Burkitt lymphoma (B-cell lymphoma) | Not performed | None | no |
| Aslam et. al. 9 | ~47–48 | 46 | Peripheral T-cell lymphoma | Not reported | Trisomy 21 | no |
| Yiannias et. al. 10 | ~32–40 | 45 | Peripheral T-cell lymphoma | Positive anti-Smith, dsDNA | Lympho-histiocytic panniculitis | no |
AMA, anti-mitochondrial antibody; ANA, anti-nuclear antibody; C3, complement component 3; C4, complement component 4; ENA, extractable nuclear antigens; GAD, anti-glutamic acid decarboxylase antibody; RF, rheumatoid factor; TC, total complement; TG, anti-thyroglobulin antibody; TPO, anti-thyroid peroxidase antibody
Case 1
A 63 year old Caucasian man noted generalized loss of subcutaneous fat over several months. Concomitantly he developed diabetes with extreme insulin resistance (requiring 3–4 units/kg/day of insulin), hypertriglyceridemia, elevated transaminases, and hepatosplenomegaly with steatohepatitis on liver biopsy. He had leukopenia (white blood cell count [WBC] 2000/μL) with relative neutropenia. Lymph node and bone marrow biopsies were non-diagnostic, and he was diagnosed with chronic idiopathic neutropenia. Repeat bone marrow biopsy four years later showed marked hypercellularity with erythroid predominance and reactive lymphoid nodules. CBC showed moderate leukopenia, anemia, and mild thrombocytopenia. At initial NIH evaluation two weeks later, his WBC count was 2880/uL with an absolute neutrophil (ANC) count of 514/uL, hemoglobin 12.2 g/dL, and platelets 102,000/uL. Hemoglobin A1c (HbA1c) was 8.1%, fasting glucose 149 mg/dL, insulin 109 μU/mL, triglycerides 220 mg/dL, ALT 128 U/L, AST 79 U/L, and liver biopsy showed steatohepatitis. Autoimmune markers were negative (Table I). Physical examination demonstrated diffuse lymphadenopathy and a 2–3 cm purplish skin lesion on a lower extremity. Metreleptin treatment was initiated, and shortly thereafter his local hematologist started GCSF for neutropenia.
At month eight of metreleptin, his metabolic status was improved, with HbA1c 6.8%, decreased insulin doses, fasting insulin 54 uIU/mL, and triglycerides 168 mg/dL. At that time, the patient reported a 30-day history of worsening skin lesions on the right leg. Biopsy revealed peripheral T-cell lymphoma (PTCL, Figure 1A and 1B). Epstein-Barr Encoded RNA (EBER) probe was negative. Bone marrow biopsy showed an atypical T-cell infiltrate suggestive of PTCL. Metreleptin was discontinued. The patient died from PTCL 6 months after diagnosis.
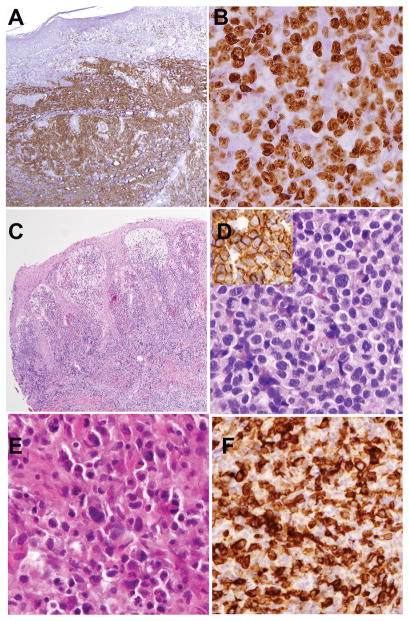
Figure 1.
Pathological findings of T-cell lymphomas arising in three patients with acquired generalized lipodystrophy.
Case 1. Peripheral T-cell lymphoma, NOS. Atypical lymphoid cells formed a tumor on the right lower leg. The atypical lymphoid cells replaced the dermis and extended into subcutaneous tissue. There was no epidermotropism. A. A stain for CD5 highlights the extent of infiltration. The neoplastic cells were also positive for CD3, CD4 and Beta F1, and negative for CD8 and CD30 (not shown). Cytotoxic molecules were negative. B. Nearly all cells are positive for MIB-1(Ki-67), indicating a high proliferative rate. Cells have large round to irregular nuclei and distinct basophilic nucleoli.
Case 2. Peripheral T-cell lymphoma, NOS, involving the skin of ankle C. Neoplastic cells extend from superficial dermis to subcutis. There is superficial edema but minimal epidermotropism. D. At higher power, neoplastic lymphoid cells are round to oval in shape with clear cytoplasmic borders. Cells are CD3-positive (Inset), and were also positive for CD5 (weak), CD4 and Beta F1; cells were negative for CD8, CD30, and TCR-gamma
Case 3. ALK-positive anaplastic large cell lymphoma. E. A soft tissue mass from the region of the right breast shows infiltration by atypical, pleomorphic cells with a range in cell size. F. The cells are strongly positive for CD30, and showed nuclear and cytoplasmic staining for ALK (not shown).
Original magnifications: A, C, 20x; B, D, E, F, 400x.
Case 2
A Hispanic woman developed gradual loss of subcutaneous fat in the 5th decade of life, resulting in generalized lipodystrophy. In conjunction with fat loss, she developed diabetes, hypertriglyceridemia, and hepatic steatosis. At age 58 years, she developed neutropenia, and GCSF was initiated. Bone marrow biopsy performed during GCSF treatment showed hypercellularity with marked atypical T-cell lymphocytosis and myeloid maturation with left shift. At initial evaluation at NIH (age 59 years), WBC and ANC counts were normal on GCSF (7240/uL and 2158/uL, respectively), absolute lymphocyte count was elevated at 4293/uL, hemoglobin was 11.8 g/dL, and platelet count 170,000/uL. Autoimmune markers were negative (Table I). Her fasting glucose was 205 mg/dL, insulin 49.1 uIU/mL, HbA1c 7.9%, and triglycerides 268 mg/dL. Metreleptin was initiated.
Four months after starting metreleptin, the patient was diagnosed with multifocal intraductal carcinoma of the right breast without lymph node invasion, and treated with mastectomy and tamoxifen. Eight months after starting metreleptin, she developed a nodular skin rash (Figure 2). Biopsies obtained from cutaneous thick plaques and nodules from the ankle, lower leg, and right arm showed similar histological features (Figure 1C and 1D), consistent with PTCL. EBER probe was negative. Bone marrow biopsies showed an atypical lymphoid infiltrate, consistent with involvement by PTCL. Metreleptin was discontinued. The patient died from PTCL 8 months after diagnosis.
Figure 2.
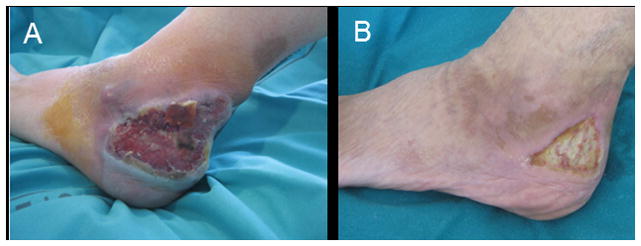
Clinical photographs of peripheral T-cell lymphoma, NOS, in a patient with acquired generalized lipodystrophy (Case 2) before (A) and after (B) chemotherapy.
Case 3
A Hispanic female born with normal body fat had progressive loss of fat between 1 and 2 years of age resulting in generalized lipodystrophy. At age 11 years, NIH evaluation revealed hypertriglyceridemia (368 mg/dL), severe insulin resistance (HbA1c 5.3%, fasting glucose 108 mg/dL, insulin 251 uIU/mL), and non-alcoholic steatohepatitis (ALT 36 U/L, AST 20 U/L) with possible cirrhosis (on liver biopsy), and she was initiated on metreleptin. She had no hematologic abnormalities (WBC 4510/μL, ANC 2255/μL, hemoglobin 13.2 g/dL, and platelets 188,000/μL), and autoimmune markers were negative (Table I). CBC at the time of leptin Labs at month 12 of metreleptin showed stable HbA1c, improved triglycerides to 253 mg/dL, and reduction in insulin level to 10.3 uIU/mL. CBC and chemistries remained normal.
After 21 months of metreleptin, the patient developed a non-tender, 5×4 cm mass in the right breast of two weeks duration that was heterogeneously enhancing on MRI. Core needle biopsy showed anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL) (Figure 1E and 1F). Molecular diagnostics showed a clonal T-cell population consistent with ALCL. EBER probe was negative. Two weeks after the needle biopsy, the mass had spontaneously reduced in size. Excisional biopsy was performed, confirming ALCL, but with a paucity of neoplastic cells. CSF and bone marrow were negative for malignant cells. Fasting insulin was 76 uIU/mL, glucose 97 mg/dL, and triglycerides 235 mg/dL. Metreleptin was discontinued. One month after the excisional biopsy, fasting insulin was increased to 398–675 μIU/mL, glucose was 125–158 mg/dL (after metformin was held for 48 hours), triglycerides 385 mg/dL, ALT 175 U/L, and AST 83 U/L. MRI showed no residual disease. After discussions with oncology consultants and the IRB, metreleptin was restarted 6 weeks after the excisional biopsy, as the benefits of metreleptin for the patient’s metabolic disease were felt to be substantial, whereas the risks of metreleptin in relation to lymphoma were considered theoretical. In addition, there was concern that the patient would be unable to tolerate chemotherapy with glucocorticoids and hepatotoxic drugs without metreleptin. PET performed three months after excisional biopsy showed no evidence of residual disease. Chemotherapy with vincristine, doxorubicin, Cytoxan, and prednisone (protocol POG9219) was administered, and was well-tolerated with the exception of glucocorticoid-induced diabetes requiring U-500 insulin. PET performed nine months after chemotherapy showed no residual disease.
Case 4
A 35 year-old woman developed red-brown patches on the left lateral breast and the groin bilaterally. Biopsy demonstrated poikiloderma atrophicans vasculare (poikilodermatous mycosis fungoides). The lesions persisted during treatment with only nutritional therapies, and repeat biopsy four years later showed evidence of persistent disease. Immunophenotypic studies showed a CD3+/CD4+/CD8− immunophenotype, with partial loss of CD7, consistent with mycosis fungoides. The skin lesions subsequently reduced substantially with only natural therapy (visual imagery and herbal treatments), and the mycosis fungoides was considered resolved. Over 25 years later (age 60), the patient noted rapid loss of fat after a flu-like illness. Shortly thereafter she was diagnosed with hypertriglyceridemia (>4000 mg/dL) and diabetes (HbA1c > 7.5%). Evaluation revealed generalized lipodystrophy and a low leptin level of 1.8 ng/ml. Insulin (0.3 U/kg) was used to control her diabetes (HbA1c maintained less than 8%) and fish oil to maintain triglycerides <1000 mg/dL. At age 68, she developed anemia (hematocrit 33.6%) and increased abdominal girth. Abdominal ultrasound revealed ascites, which was found to contain adenocarcinomatous cells of undetermined origin. She was treated with metreleptin for 2.6 months under an expanded access protocol (NCT00677313) with improved triglycerides (to <400 mg/dL) and diabetes control (HbA1c < 7%) off insulin. During her treatment with metreleptin, there was no evidence of recrudescence of the mycosis fungoides. She declined treatment for the adenocarcinoma, discontinued metreleptin, and died from adenocarcinoma soon after.
Case 5
A 16-yr-old boy was referred for diabetes, hypertriglyceridemia, acanthosis nigricans and hepatomegaly with elevated liver enzymes in the context of generalized lipoatrophy. AGL was diagnosed on the basis of late childhood onset of lipoatrophy after a pulmonary infection, and negative testing for genetic causes of lipodystrophy. Diffuse bone and muscular pain with fever rapidly occurred. Physical examination revealed splenomegaly, and CT showed mediastinal and mesenteric lymphadenopathy. Laboratory investigations demonstrated inflammation (elevated high-sensitivity C-reactive protein and fibrinogen) and anemia. Bone marrow aspiration showed 95% lymphoblast infiltration that expressed CD19, CD10, CD20, CD22, surface light chain lambda and IgM, and TdT. The bone marrow karyotype was 46,XY,add(14)(q32)[12]/46,XY[28]. Fluorescence in situ hybridization showed an atypical IGH/MYC rearrangement that in conjunction with a mature B-cell phenotype favored the diagnosis of Burkitt lymphoma, although the diagnosis of acute lymphoblastic leukemia could not be ruled out based on TdT rearrangement. Complete remission was obtained after chemotherapy, but lipoatrophy and insulin-requiring diabetes were unchanged after 18 months of follow-up.
DISCUSSION
This case series demonstrates an association between AGL, a very rare disorder resulting in generalized loss of adipose tissue, and lymphoma, particularly peripheral T-cell lymphoma. The current report adds to two previously published cases of PTCL in patients with AGL. Aslam et al. reported a 46 year old man with trisomy 21 who developed PTCL, followed 18 months later by progressive loss of fat resulting in generalized lipodystrophy9. The loss of fat was accompanied by typical metabolic manifestations of lipodystrophy, including diabetes with severe insulin resistance, hyperphagia, and severe hypertriglyceridemia. Yiannias et. al. reported a 32 year old man who developed panniculitis followed by gradual loss of fat over 7 years leading to generalized lipodystrophy, accompanied by hypertriglyceridemia and increased ALT/AST10. Thirteen years later, the patient developed PTCL. An additional case of Hodgkin’s lymphoma was reported in a patient with generalized lipodystrophy and scleroderma who was never treated with metreleptin, however, the presence or subtype (acquired versus genetic) of lipodystrophy could not be verified11. The absolute risk of lymphoma in AGL is difficult to estimate due to the rarity of AGL (~100 cases reported1). However, within the NIH cohort, the prevalence of lymphoma in AGL patients was 18% (3 of 17 patients), suggesting a substantially increased risk relative to the general population (2.09 per 100,000 persons12). Lymphoma has not been reported in genetic forms of lipodystrophy, thus risk for lymphoma appears to be a feature of AGL rather than associated with lipodystrophy per se.
The coexistence of AGL and lymphoma likely relates to underlying autoimmunity. Autoimmune diseases occur commonly in patients with AGL1,3,13–15, including both organ-specific autoimmunity (e.g. type 1 diabetes, autoimmune hepatitis) and systemic autoimmune diseases (e.g. juvenile dermatomyositis)16. Even in the absence of documented autoimmunity (i.e. positive autoimmune markers or other autoimmune diseases), AGL is thought to represent an autoimmune disease in and of itself, based on the theory that autoantibodies against adipocytes lead to destruction of fat. The epitope targeting adipocytes for destruction has not been established, and only a single case of an autoantibody to adipocytes in a patient with AGL has been reported17. Autoimmune diseases also have a known association with malignant lymphoproliferative disorders18, thus providing a plausible link between lipodystrophy and lymphomas. In large cohort studies, individuals with autoimmune diseases have an increased risk of non-Hodgkin’s lymphoma, although the absolute risk of lymphoma remains small19,20.
AGL patients appear to be at particular risk for T-cell lymphomas (especially PTCL), which are rare as compared to B-cell lymphomas. Lymphoma risk in most autoimmune diseases is associated with autoantibody production, with a specific risk for B-cell lymphoma20. PTCLs comprise a heterogeneous group of hematological malignancies originating from mature T cells, and are generally aggressive. A subtype of PTCL localized to the subcutaneous fat, termed subcutaneous panniculitis-like T-cell lymphoma, could represent a pathophysiologic link between the development of AGL, and T-cell lymphomas. Panniculitis may be the presenting feature of AGL, as in the patient with AGL and PTCL reported by Yiannias and colleagues10. Several forms of mature T-cell malignancy were encountered in our series: PTCL, not otherwise specified (n=2), mycosis fungoides (n=1), and ALCL, ALK-positive (n=1). Interestingly, the latter patient had spontaneous regression of her disease, although chemotherapy ultimately was instituted. Rare cases of spontaneous regression in ALK-positive systemic ALCL have been reported21. This patient also had an extremely rare ALK-positive localized ALCL that behaves more like primary cutaneous ALCL with good prognosis22.
Because three patients developed lymphoma during metreleptin treatment, and leptin receptors are expressed on T cells, there is concern that metreleptin could play a role in lymphoma development. Starvation leads to low leptin levels, and leptin replacement reverses starvation-induced immunosuppression in mice23. Furthermore, absolute leptin deficiency in humans due to leptin gene mutations has been associated with early mortality from infections24. Despite this, increased susceptibility to infections is not a major feature of lipodystrophy. Lipodystrophy patients do have a subtle alteration of the immune phenotype, with a decrease in certain T-lymphocyte subpopulations in the leptin-deficient state that normalize with leptin replacement25. Furthermore, while leptin differentially regulated naïve and memory CD4+ T cells, leptin in vitro had little effect on proliferation of established human T cell clones23. Thus while leptin may have a role in regulating normal T cell subsets, it is not clear whether these findings can be extended to suggest that metreleptin would promote malignant behavior. No genotoxicity of metreleptin has been demonstrated in standard in vitro or in vivo assays, and no metreleptin-related neoplastic or preneoplastic lesions were found in 6-month repeated dose toxicity studies in mice and dogs at exposures up to 17-fold the maximum clinical dose 26. Carcinogenicity studies were not conducted for metreleptin, as historically they are not relevant for biotechnology-derived products.
Lymphomas in AGL occur both in the presence (Cases 1–3) and absence (Cases 4–5 and two prior publications9,10) of metreleptin treatment, supporting the concept that metreleptin may not play a primary role in lymphoma development. Moreover, Cases 1 and 2, who developed PTCL while on metreleptin, had significant hematologic abnormalities prior to initiating metreleptin. This suggests that these patients may have had early lymphoma or a precursor condition prior to starting metreleptin.
In summary, AGL is associated with high risk for lymphoma. Lymphomas have occurred in AGL with or without metreleptin, supporting the idea that metreleptin did not play a direct causal role in lymphoma development. However, a theoretical role of metreleptin in tumor growth cannot be ruled out. For most patients with AGL and severe metabolic manifestations of lipodystrophy, the proven benefits of metreleptin on metabolic disease will likely outweigh the theoretical risks of metreleptin in development or progression of lymphoma. Optimal screening for lymphoma in AGL has not been established. In the absence of evidence of benefit, potentially harmful screening (especially involving the use of ionizing radiation) should be avoided. Patients should be educated about the risks of lymphoma and promptly report suspicious skin lesions or enlarged lymph nodes to their providers. If lymphoid malignancies develop, patients with lipodystrophy may be at greater than average risk for certain chemotherapy-related toxicities, such as steroid-induced diabetes, or hepatoxicity.
Acknowledgments
This work was supported by the intramural research programs of the National Institute of Diabetes and Digestive and Kidney Diseases (RJB, EC, PG) and the National Cancer Institute (ESJ), and by Assistance Publique-Hôpitaux de Paris and Inserm (JFG, CG and CV). Alan Wayne (NIH) Ruchika Goel (NIH), Nirali N. Shah (NIH), and Stephanie Massaro (Yale University) provided clinical care for Case 3. For case 5, Baz Baz (AP-HP) provided clinical care, Nicolas Boissel (AP-HP) gave information on lymphoma diagnosis, and Olivier Lascols (AP-HP) performed genetic tests. Wyndham Wilson provided insight in the pathophysiology of lymphoma.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
Dr. Brown has nothing to disclose. Dr. Chan reports being an employee of and holding stock in Bristol Myers Squibb, outside the submitted work. Dr. Jaffe has nothing to disclose. Ms. Cochran has nothing to disclose. Dr. DePaoli reports a patent null issued. Dr. Vigouroux reports personal fees from AstraZeneca outside the submitted work. Dr. Goujard has nothing to disclose. Dr. Gorden has nothing to disclose.
References
- 1.Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87(5):2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 3.Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17(6):922–932. doi: 10.4158/EP11229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53(1):27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 5.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 6.Park JY, Javor ED, Cochran EK, DePaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism. 2007;56(4):508–516. doi: 10.1016/j.metabol.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92(2):532–541. doi: 10.1210/jc.2006-1546. [DOI] [PubMed] [Google Scholar]
- 8.Araujo-Vilar D, Sanchez-Iglesias S, Guillin-Amarelle C, et al. Recombinant human leptin treatment in genetic lipodystrophic syndromes: the long-term Spanish experience. Endocrine. 2014 doi: 10.1007/s12020-014-0450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslam A, Savage DB, Coulson IH. Acquired generalized lipodystrophy associated with peripheral T cell lymphoma with cutaneous infiltration. Int J Dermatol. 2013 doi: 10.1111/ijd.12185. [DOI] [PubMed] [Google Scholar]
- 10.Yiannias JA, DiCaudo DJ, Maskin E. Peripheral T-cell lymphoma presenting as lipoatrophy and nodules. Int J Dermatol. 2006;45(12):1415–1419. doi: 10.1111/j.1365-4632.2006.02888.x. [DOI] [PubMed] [Google Scholar]
- 11.Hall SW, Gillespie JJ, Tenczynski TF. Generalized lipodystrophy, scleroderma, and Hodgkin’s disease. Arch Intern Med. 1978;138(8):1303–1304. [PubMed] [Google Scholar]
- 12.Wang S, Vose J. Epidemiology and Prognosis of T-Cell Lymphoma. In: Foss F, editor. T-Cell Lymphomas. Humana Press; 2013. pp. 25–39. [Google Scholar]
- 13.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 14.Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 2003;82(2):129–146. doi: 10.1097/00005792-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Savage DB, Semple RK, Clatworthy MR, et al. Complement abnormalities in acquired lipodystrophy revisited. J Clin Endocrinol Metab. 2009;94(1):10–16. doi: 10.1210/jc.2008-1703. [DOI] [PubMed] [Google Scholar]
- 16.Bingham A, Mamyrova G, Rother KI, et al. Predictors of acquired lipodystrophy in juvenile-onset dermatomyositis and a gradient of severity. Medicine (Baltimore) 2008;87(2):70–86. doi: 10.1097/MD.0b013e31816bc604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubler A, Abendroth K, Keiner T, et al. Dysregulation of insulin-like growth factors in a case of generalized acquired lipoatrophic diabetes mellitus (Lawrence Syndrome) connected with autoantibodies against adipocyte membranes. Exp Clin Endocrinol Diabetes. 1998;106(1):79–84. doi: 10.1055/s-0029-1211955. [DOI] [PubMed] [Google Scholar]
- 18.Smedby KE, Askling J, Mariette X, Baecklund E. Autoimmune and inflammatory disorders and risk of malignant lymphomas--an update. J Intern Med. 2008;264(6):514–527. doi: 10.1111/j.1365-2796.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 19.Ekstrom Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029–4038. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallah M, Liu X, Ji J, Forsti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. 2014;25(10):2025–2030. doi: 10.1093/annonc/mdu365. [DOI] [PubMed] [Google Scholar]
- 21.Kedar A, Braylan RC. Lymphohistiocytic anaplastic large cell lymphoma stage I: long-term survival after resection alone. Pediatr Hematol Oncol. 2000;17(3):261–268. doi: 10.1080/088800100276442. [DOI] [PubMed] [Google Scholar]
- 22.Oschlies I, Lisfeld J, Lamant L, et al. ALK-positive anaplastic large cell lymphoma limited to the skin: clinical, histopathological and molecular analysis of 6 pediatric cases. A report from the ALCL99 study. Haematologica. 2013;98(1):50–56. doi: 10.3324/haematol.2012.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 24.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 25.Oral EA, Javor ED, Ding L, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab. 2006;91(2):621–628. doi: 10.1210/jc.2005-1220. [DOI] [PubMed] [Google Scholar]
- 26.Endocrinologic and Metabolic Drugs Advisory Committee Briefing Document for metreleptin (BLA STN125390) 2013:29. [Google Scholar]