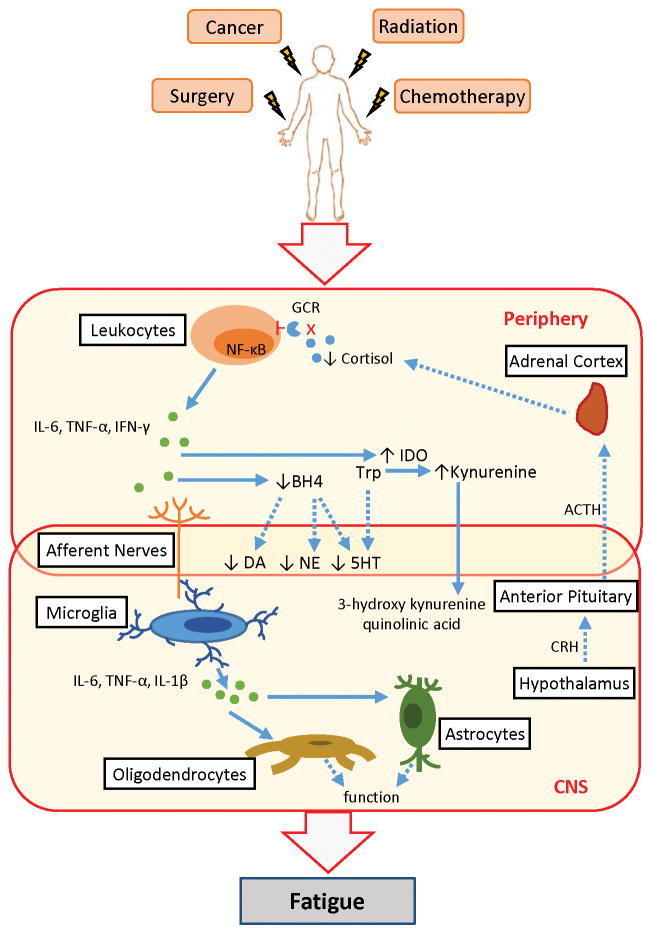
Figure 1.
Systemic inflammation contributes to cancer-related fatigue. Cancer and its treatments activate leukocytes, resulting in increased expression of the transcription factor NF-κB and production of pro-inflammatory mediators, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ). Dysregulation of the hypothalamic-pituitary-adrenal (HPA)-axis leads to altered release of cortisol; glucocorticoid receptor (GCR) function on leukocytes is also decreased. Inflammation in the periphery leads to inflammation in the central nervous system (CNS) through several immune-to-brain communication pathways. Signals from sensory nerves activate CNS cells such as microglia to produce pro-inflammatory cytokines, including IL-6, TNF-α, and interlukin-1 beta (IL-1β). These inflammatory mediators then impact neurons directly or indirectly through altered oligodendrocyte, astrocyte, and endothelial cell functions. Inflammation in the periphery also decreases the availability of precursors, such as tetrahydrobiopterin (BH4) required for the synthesis of the neurotransmitters dopamine (DA), norepinephrine (NE), and serotonin (5HT). Deficits in serotonin production also occur as tryptophan (Trp) is converted into kynurenine from an inflammation-mediated increase in indoleamine 2,3-dioxygenase (IDO). Kynurenine is further metabolized in the brain into neurotoxic kynurenine metabolites such as 3-hydroxy kynurenine and quinolinic acid. Dashed lines indicate a disrupted pathway. CRH: corticotropin release hormone; ACTH: adrenocorticotropic hormone