Summary
Natural immunoglobulin derived from innate-like B lymphocytes plays important roles in the suppression of inflammatory responses and represents a promising therapeutic target in a growing number of allergic and autoimmune diseases. These antibodies are commonly autoreactive and incorporate evolutionarily conserved specificities, including certain glycan-specific antibodies. Despite this conservation, exposure to bacterial polysaccharides during innate-like B lymphocyte development, through either natural exposure or immunization, induces significant changes in clonal representation within the glycan-reactive B cell pool. Glycan-reactive natural antibodies have been reported to play protective and pathogenic roles in autoimmune and inflammatory diseases. An understanding of the composition and functions of a healthy glycan-reactive natural antibody repertoire is therefore paramount. A more thorough understanding of natural antibody repertoire development holds promise for the design of both biological diagnostics and therapies. In this article we review the development and functions of natural antibodies and examine three glycan specificities, represented in the innate-like B cell pool, to illustrate the complex roles environmental antigens play in natural antibody repertoire development. We also discuss the implications of increased clonal plasticity of the innate-like B cell repertoire during neonatal and perinatal periods, and the prospect of targeting B cell development with interventional therapies and correct defects in this important arm of the adaptive immune system.
Keywords: Natural Antibody, innate-like B lymphocyte, B-1 B cell, Repertoire development, Glycan neodeterminant, Clonotype
Introduction
Among white blood cells, the B-lymphocyte (B cell) pool consists of several B cell populations (cellular compartments or subsets) that segregate developmentally, anatomically, and functionally: the B-1 B cells, Marginal Zone (MZ) B cells, and Follicular (FO) B cells. Each of these B cell subsets perform distinct and specialized functions in host immunity, forming part of what has been termed a layered immune system (1). FO B cells cooperate with T lymphocytes to produce high-affinity, class-switched antibody responses. The innate-like B cells, B-1 and Marginal Zone B cells, promote rapid capture and neutralization of antigen through T lymphocyte-independent (T-independent) antibody responses (2). T-independent responses include "natural" antibodies, which are produced in the absence of deliberate immunization. This review focuses on the development of polysaccharide-reactive B-1 B cells, natural antibodies, and how these antibodies, which contribute to immunological homeostasis, can be manipulated for immunotherapies.
In addition to their contributions to host-defense against a variety of fungal, bacterial, and viral pathogens (3), B-1 B cells assist in the maintenance of immunological homeostasis by producing antibodies that significantly suppress allergic and systemic autoimmune disease development (4, 5). Importantly, neonatal antigen exposure drives alterations in the B cell repertoire that manifest as clonally and functionally distinct adult antibody responses (5). Thus, clonal specificities represented in the adult B cell repertoire result from a temporal interplay between B cell developmental programs and antigenic exposure. Given the multiple roles of nAbs, the contribution of external influences to B-1 B cell clonal selection and natural antibodies (nAbs) repertoire development is a significant, yet poorly understood topic.
A central theme in this article will be the evaluation of effects of exogenous antigen stimulation on B cell repertoire development. After observing that rates of childhood hay fever and eczema were lower in larger households relative to smaller households, Strachan proposed in 1989 that exogenous antigen stimulation benefited efficacious immune system development. In what became known as the Hygiene Hypothesis, Strachan reasoned that atopic diseases may be prevented by infections in early childhood (6). Consistent with this theory, the global prevalence of many allergic and autoimmune diseases inversely correlates with that of various human pathogen infections, and developed societies are experiencing rapid increases in the rates of incidence of these diseases (7–9). These trends generally correlate with improvements in household amenities and personal cleanliness standards, compounded with liberal antibiotic use in developed societies and reduced parasite burden. The increased incidence of aberrant immunological phenomena in developed societies may therefore result from lowered exogenous antigen exposure during the perinatal period, resulting in an inappropriate immune system development. Although much emphasis has been placed on a role for these antigens in altering T helper lymphocyte (TH1/TH2) balance, the effects on the development of the B cell repertoire are still largely unknown.
This review integrates our understanding of (i) natural antibody immunomodulatory functions, (ii) genetic constraints on innate-like B cell development, (iii) the impact of neonatal antigen experience on clonal representation, and (iv) antibody repertoire plasticity during early life. Our goal will be to illustrate the therapeutic potential of this arm of the adaptive immune system in suppressing allergic and autoimmune diseases. We specifically focus on the development and function of glycan-specific natural antibodies, sharing our own primary findings on the physiological implications of natural antibodies that recognize autologous, cryptic glycan epitopes (molecular structures on each antigen where the antibody binds). Lastly, we discuss the future of natural antibody research, as well as the potential of natural antibody-based diagnostic tools and therapies
The Development and Functions of Natural Antibodies
Natural or preimmune antibody is defined as antibody existing in serum without deliberate immunization, and is predominantly of the IgM isotype (antibody class). Serum IgM levels are tightly regulated, and are quantitatively independent of exposure to exogenous antigen (10). Natural antibodies are the first-line-of-defense for the adaptive immune system against invading pathogens (Figure 1A). This significant function of IgM is made apparent by the increased susceptibility to bacterial infection displayed by both secretory IgM-deficient (sIgM−/−) mice, and by humans harboring rare disorders resulting in IgM deficiency (11–13). Natural IgM provides immunity against many bacterial, viral, and fungal pathogens (14–18), and is a critical protective component of the immediate immune response. In cecal-ligation and puncture models of sepsis, reconstitution of polyclonal serum IgM significantly reduces the mortality of sIgM−/− mice (19). Orthologous epitopes, or related epitopes found on different species, exist in diverse forms and allow natural IgM of a single specificity to protect against multiple pathogens (3, 20). The configuration, density, and three-dimensional array of these epitopes all influence the affinity and avidity of epitope recognition by IgM, which in turn affects the function of IgM antibodies.
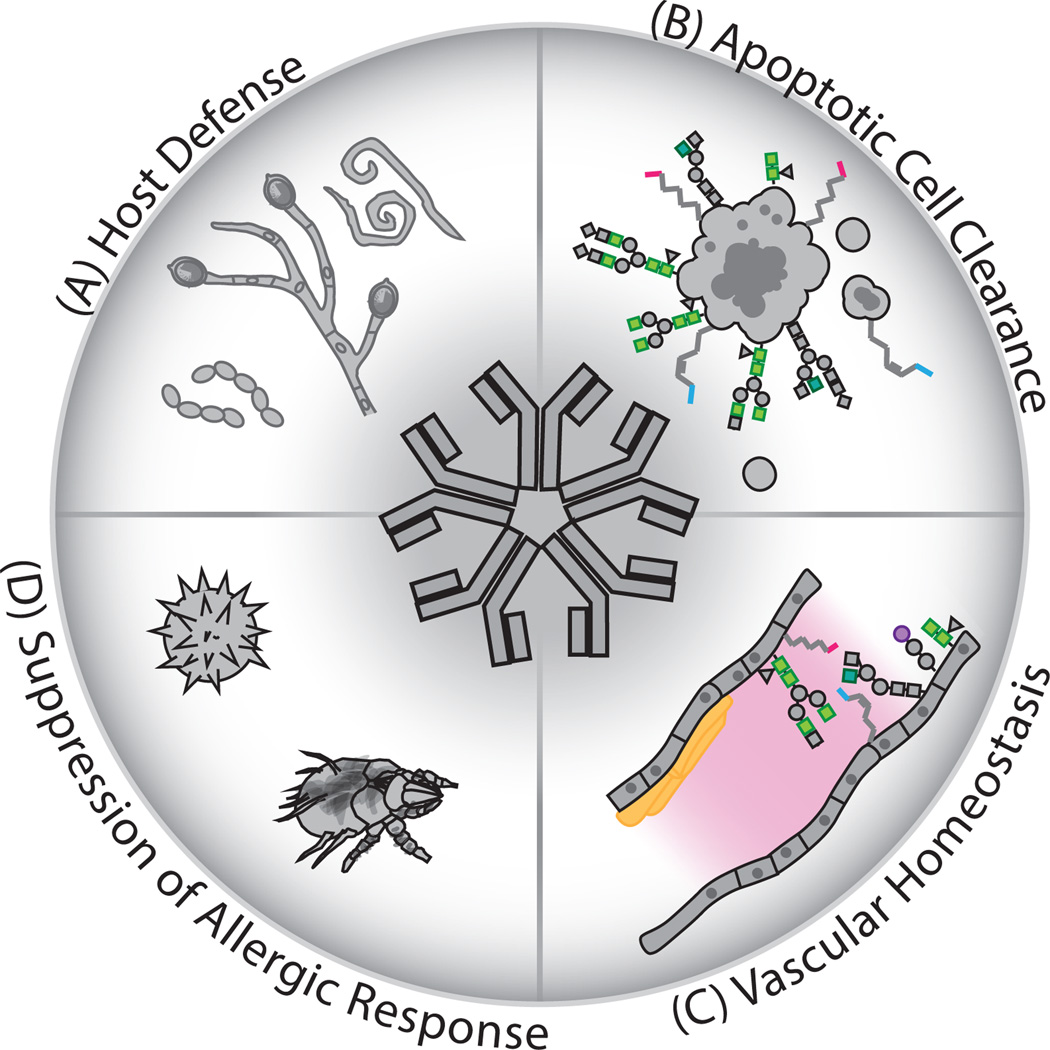
Figure 1. Natural antibodies fulfill multifunctional roles in host immunity.
Pentameric IgM natural antibodies perform several important functions in immunological homeostasis. (A) Natural IgM is an important first-line-of-defense against invading pathogens, implicated in protection against infection by bacterial, viral, fungal, and multicellular pathogens including helminths. (B) The specificity of these antibodies also includes reactivity toward certain autologous antigens. Natural antibodies facilitate the efficacious clearance of apoptotic cells through recognition of danger-associated molecular patterns generated during apoptosis, such as phospholipid and glycan neoantigens. (C) Natural Ab-reactive neoantigens are generated on the vascular endothelium following ischemia reperfusion injury and nAbs suppress inflammatory lesions associated with deposition of atheroscerlotic plaques. (D) Natural Abs additionally suppress sensitization to allergens through reactivity with conserved T lymphocyte-independent epitopes present on allergenic particulates. Following opsonization of target antigens, nAbs can alter phagocyte activation and reduce the resulting T lymphocyte-dependent responses necessary for production of pathogenic IgG autoantibodies or mast-cell sensitizing IgE.
Some of these antigenic epitopes are also normal constituents of mammalian cells or tissues, and natural antibodies (nAb) promote immune homeostasis and suppress autoimmunity through the recognition of these autologous antigens (Figure 1B) (4). These effects have been linked to nAb recognition of apoptosis-associated neodeterminants (newly appearing structures), such as lysophosphotidylcholine phospholipid head groups, that are exposed on senescent cells during normal apoptotic processes that disrupt membrane asymmetry (21–24). Opsonization (coating the apoptotic entity) with natural antibodies reactive with phosphorylcholine (PC) promotes efficacious clearance of potentially inflammatory apoptotic cells (22). Analysis of antibody responses following immunization of mice with apoptotic autologous thymocytes led to the discovery of additional oxidation-related lipid neodeterminants on apoptotic cells such as malondialdehyde, a product of ROS-mediated oxidation of polyunsaturated lipids, recognized by nAbs (25). Thus, phospholipid and oxidatized lipid neoantigens appear to be major targets of nAb specificity, and nAbs reactive with these epitopes have been implicated in various immunological processes, such as the suppression of systemic autoimmunity (4). The significance of this homeostatic mechanism is apparent in the significantly increased pathogenic IgG autoantibody titers and exacerbated immune-complex glomerulonephritis observed when secretory sIgM-deficiency (sIgM−/−) is introduced to the systemic lupus erythematosus-prone (lpr) mouse model (sIgM−/−C57BL/6.lpr) (13). In fact, sIgM-deficiency itself is sufficient to drive increased titers of autoantibodies directed against apoptotic cell-associated antigens, suuch as double-stranded DNA-specific IgG (13, 26). These findings demonstrate the importance of natural antibody-mediated apoptotic-cell clearance in immunological homeostasis and preventing aberrant immune responses towards autologous antigens.
Natural antibodies recognizing vascular endothelium-localized neodeterminants have also been described (Figure 1C). Accumulating evidence indicates that lipid- and oxidized lipid-reactive nAbs reduce tissue necrosis in atherosclerotic lesions by recognizing oxidized low-density lipoprotein molecules (27). These atheroprotective nAb functions have been reviewed in detail elsewhere (28). In ischemia-reperfusion (IR), nAbs recognize neodeterminants on the vascular endothelium following IR injury, mediate complement deposition, and drive detrimental vascular inflammation and tissue damage (29, 30).
Our lab has extended the understanding of nAb immunomodulatory functions to include allergen recognition and the suppression of allergic sensitization (Figure 1D) (5). We have reported that certain natural antibody clonotypes react with fungi- as well as mite-derived allergenic particulates, and may be induced at sufficient levels to suppress allergic sensitization through immunization with bacteria bearing homologous antigens. These antibodies therefore serve dual-protective functions facilitated by conservation of T-independent antigenic epitopes between certain infectious bacteria and allergenic antigens. Allergic sensitization to intra-tracheally administered fungal and house-dust mite allergens is significantly suppressed following neonatal immunization with polysaccharide- or phospholipid-bearing bacteria, respectively (3, 31). We also reported that intra-tracheal priming with allergens recognized by B-1 B cell clonotypes leads to recruitment of IgM-secreting B cells into the lung tissue (31), and secretion of IgM into the bronchiolar alveolar space. We find that these antibodies block recognition of potential allergens by innate-immune cells, thereby subverting sensitization to allergens.
The immunomodulatory functions of nAbs include suppression of inflammatory signaling after phagocytic recognition of immunogenic molecules by innate phagocytes. Whereas the universality of this immunomodulatory activity is unclear, many autoantigen targets of nAbs are also ligands for pattern-recognition receptors (PRRs) expressed by innate leukocytes and epithelial cells, and these receptor interactions represent a form of innate autoimmunity (32, 33). Innate receptors including integrins, scavenger receptors, and lectins have been implicated in recognizing apoptosis-associated neodeterminants and also allergens, and driving potent activation of innate-immune cells (32). Although recognition of apoptotic cells induces tolerogenic phenotypes of dendritic cells (DCs) (34), it is clear that stimulation of certain innate PRRs can drive cross-presentation of apoptotic cell-associated antigen to cytotoxic T lymphocytes (35). Furthermore, neodeterminants generated through post-translational protein modifications during apoptosis represent the major targets of pathogenic autoantibodies (36). Competition for these innate receptor ligands may partially explain how nAbs can suppress Toll-like receptor (TLR) signaling following apoptotic cell phagocytosis by dendritic cells; however, natural IgM can also directly suppress TLR-driven activation signals, through induction of MAPK1 phosphatase, and induce tolerogenic DCs (37, 38). These effects suppress T cell activation and differentiation resulting in decreased production of pathogenic, class-switched autoantibodies (39). Because nAbs mediate immune tolerance of autologous antigens, they represent an exciting research area for the development of interventional immunotherapies.
Glycan-specific natural antibodies: Specificities and Functions
Studies of canonical phopholipid-reactive natural antibody specificities have been central to the growing appreciation of the pleiotropic functions of natural antibodies. Another major surface component of mammalian cells are glycan epitopes on glycoproteins and glycolipids, however investigations into the homeostatic functions of glycan-specific nAbs are more limited than those of lipid-reactive antibodies. Nonetheless, glycan-reactive antibodies are highly represented in the circulating antibody pools of humans and mice (40, 41). In this section, we review the origins and physiological roles of glycan-reactive natural antibodies.
Global analysis of the polysaccharide (PS) specificities represented in the adult human serum IgM pool reveals the presence of antibodies reactive with numerous glycan moieties present in the mammalian glycome, including reactivity with well-studied xeno- and alloantigens (42). It is also clear that even healthy individuals possess antibodies reactive with autologous blood group antigens (43). Such autoreactive specificities likely rely on poorly understood mechanisms for their maintenance in the glycan-reactive B cell pool. Interestingly, certain glycan specificities are highly conserved in the human population, seemingly representing a set of core clonal-specificities for certain glycan epitopes with small pockets of variability (44). Furthermore, it is clear that in many cases, natural antibody B cell clonotypes are conserved in the human population, possibly due to restricted antibody heavy chain variable region (IGHV) gene usage (45–47).
In mammals, mature glycans are typically capped with sialic acid residues such as N-acetylneuraminic Acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) that confer important structural and functional changes in the modified molecules (48). By contrast, immunoreactive moieties such as N-acetyl-D-Glucosamine (GlcNAc) and Mannose (Man) are only present in the cores of these structures. Although it is clear that healthy humans and mice display low levels of serum antibody reactivity with mature autologous N-linked glycan chains, easily detectable antibodies reactive with the core components of autologous glycans are common in mammals. These antibodies are likely generated in response to conserved antigens on environmental or commensal microorganisms; however, the physiological implications of such cryptic autologous-glycan reactive B cells are unclear.
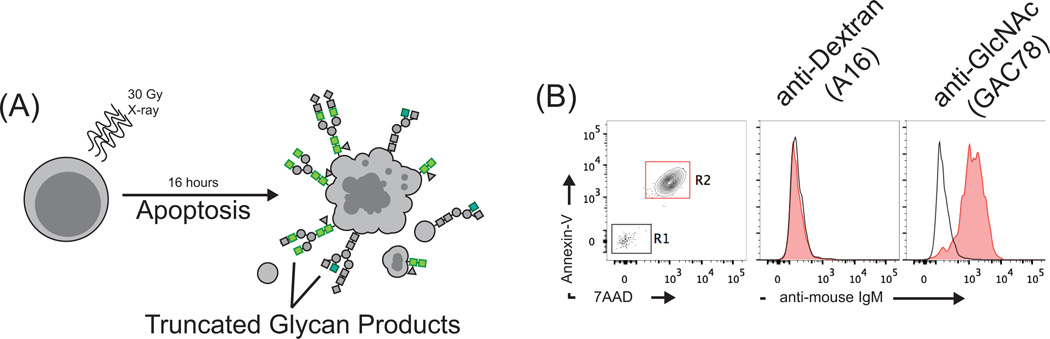
GlcNAc is the constituent monosaccharide of chitin (the second most abundant biopolymer on earth) which is a structural component of many microorganisms, and GlcNAc residues are present in cell wall antigens of many common pathogens, including Group A Streptococcus (GAS) (20, 49). In mammals, GlcNAc residues exist in several forms resulting from post-translational modification; however it is most commonly found as a cryptic epitope buried within the context of mature glycan chains (50). Similarly to the reported functions of phospholipid-specific nAbs, we have observed that anti-GlcNAc antibodies elicited by immunization with GAS bind to irradited thymocytes (Figure 2A). This reactivity is restricted to apoptotic thymocytes, correlating with positive staining for Annexin-V and 7-AAD (Figure 2B, right), and activated caspase-3 (not shown), and is not observed with isotype control antibodies specific for dextran (Figure 2B, middle). As discussed above, the structures recognized by these antibodies are commonly present in a concealed, inaccessible state within the core of N-linked glycans. These observations strongly suggest the generation or exposure of aberrantly truncated glycan epitopes occurs during apoptosis. Indeed, previous reports have implicated apoptosis-associated changes in glycan profiles, particularly decreased sialyation of surface glycans, in the phagocytic recognition of apoptotic cells (51). Furthermore, cell surface lectin-dependent recognition of both GlcNAc and fucose epitopes is important for clearing early apoptotic cells by macrophages (52–55). These apoptosis-associated neoglycans are percieved as immunogenic epitopes due to highly redundant serum lectin-opsonins and cell-surface receptors specific for immunoreactive PS-structures.
Figure 2. GlcNAc-specific natural antibodies recognize apoptosis-associated neoepitopes generated on irradiated thymocytes.
(A) C57BL/6 thymocytes were irradiated to induce apoptosis, cultured overnight, then stained with Annexin-V, the membrane impermeable DNA dye 7-AAD, and for glycan neodeterminants using various polysaccharide-reactive monoclonal mouse antibodies for flow cytometric analysis. (B) Annexin-V and 7-AAD staining (left) were used to discriminate between live (R1, black, Annexin-V−,7AAD−) and apoptotic thymocytes (R2, red, Annexin-V+,7AAD+). Binding analysis of a GlcNAc-specific monoclonal mouse antibody induced by immunization with Group A Streptococcus (GAC78, right) reveals strong reactivity with apoptotic but not live thymocytes. A mouse monoclonal antibody specific for dextran (A16, middle) shows no reactivity. These observations strongly suggest that immunoreactive cryptic GlcNAc epitopes are accessible to antibodies during apoptotic processes.
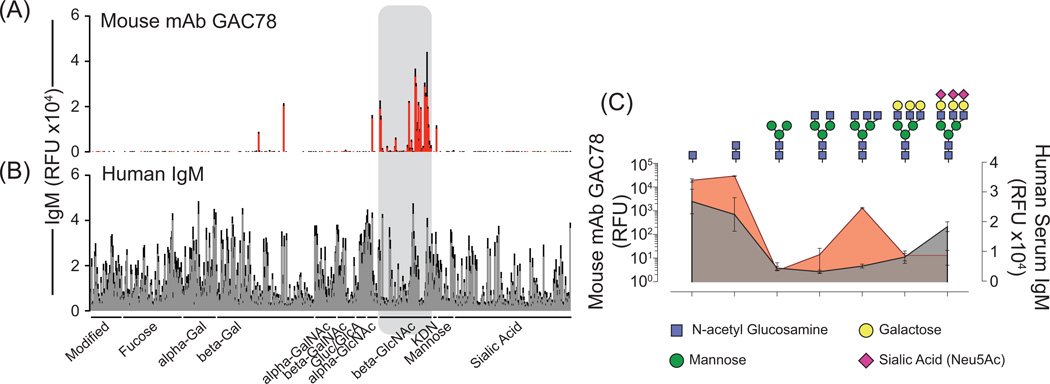
We have assayed the fine autoreactive specificity of antibodies generated in response to GlcNAc-moieties of the Group A Carbohydrate (GAC) by glycan microarray analysis (Consortium for Functional Glycomics Emory University, Atlanta, Georgia), and found that GAC-specific mAbs preferentially react with mammalian GlcNAc-β-1,4- and GlcNAcβ-1,6- terminating glycans (Figure 3A). To illustrate the potential physiological functions of GlcNAc-specific nAbs in recognizing apoptotic-cell associated glycosylation profiles, we have formatted our glycan microarray data to show reactivity for each of the constituent components of a mature glycan isolated from human pancreas and identified by mass spectrometry (CFG). As depicted in Figure 3C (red), GAC78 displays robust reactivity toward GlcNAc-terminating precursor glycan structures, particularly with the β-monomeric and dimeric GlcNacβ-1,4-GlcNAcβ core of N-linked glycans. Additional lower levels of reactivity are observed with more complex branching structures terminating with GlcNAcβ-1,6-(GlcNAcβ-1,2-)Mannoseα-1,3- and GlcNAcβ-1,4-(GlcNAcβ-1,2-)Mannoseα-1,3- structures. Importantly, these structures are rarely, if ever, exposed in mature glycan forms, and reactivity with these epitopes is restricted to those present as terminal glycan residues and is not observed in the context of the mature glycan chain.
Figure 3. Mouse antibodies elicited by immunization with Group A Streptococcus, and polyclonal human serum IgM recognize aberrantly truncated mammalian glycans.
(A) Analysis of the fine-specificity exhibited by the mouse monoclonal antibody GAC78 for mammalian glycans by glycan microarray reveals specific reactivity with beta-GlcNAc-terminating glycans. Highest affinity was observed for β-1,4-, β-1,6- and monomeric β-GlcNAc structures. (B) Open source mammalian glycan array data examining polysaccharide autoreactivity represented in polyclonal human serum IgM derived from healthy donors (n=9) demonstrates wide-spread recognition of epitopes contained within the mammalian glycome. IgM reactivity for GlcNAc-terminating glycans was observed in all human serum samples analyzed. (C) Glycan microarray results were formatted to emphasize reactivity for the constituent glycan structures of a mature human glycan identified in pancreatic tissue by mass spectrometry. (top) Illustrations depicting the glycan structures for which respective binding, measured by relative fluorescence units, was obtained following microarray analysis are shown (bottom). Mouse mAb GAC78 (red) exhibits high reactivity for the chitobiose (GlcNAcβ-1,4-GlcNAcβ) core elements, as well as reactivity for terminal branching structures of GlcNAcβ-1,6-(GlcNAcβ-1,2-)Mannoseα-1,6- and GlcNAcβ-1,4-(GlcNAcβ-1,2-)Mannoseα1–3 (not shown). β-1,2-GlcNAc epitopes are not recognized by GAC78, nor are GlcNAc epitopes contained within the context of a mature glycan chain; reactivity is associated only with glycans terminating in particular configurations of GlcNAc. Polyclonal serum IgM from healthy human donors (grey) also exhibits reactivity with chitobiose core elements; however, reactivity for GlcNAcβ-1,6-(GlcNAcβ-1,2-)Mannoseα-1,6-structures was not detected in these samples. Collectively, these results indicate that natural IgM reactivity for cryptic glycan epitopes in mammalian N-linked glycan chains are conserved between mice and humans.
GlcNAc-specific antibodies are common in the human population (49). Therefore, using open-source microarray data from the Consortium for Functional Glycomics (data generated by Dr. Nicolai Bovin, Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Moscow, Russian Federation), we analyzed the conservation of antibody reactivity with truncated glycan products in healthy human serum. As previously reported, the IgM fraction of human sera displays broad recognition of various mammalian polysaccharides, including sialic-acid terminating glycans (Figure 3B). Compared to the constituent glycan-reactivity profiles of the mouse monoclonal antibody GAC78, polyclonal human serum IgM antibodies demonstrate similar recognition profiles for GlcNAcβ-1,4- truncated N-linked glycan products (Figure 3C, grey). Thus, glycan microarray data sets indicate that healthy individuals commonly possess IgM antibodies reactive with cryptic epitopes within autologous glycans.
We have identified wide-spread immune system-related implications of antibody recognition of GlcNAc-terminating glycan neodeterminants. In addition to their recognition of apoptotic cell membranes, GlcNAc-specific natural antibodies modulate complement deposition on the surfaces of apoptotic cells and exhibit suppressive effects on Lectin-dependent complement pathway activation (JSN and JFK, manuscript in preparation). We further found that GlcNAc-specific nAbs bind ligands on subsets of insulin-containing secretory granules in pancreatic beta cells which may result from aberrant post translational modifications. These antibodies appear to play a role in suppressing inflammatory signals from phagocytic recognition of beta cell-derived autoantigens and the progression of Type 1 Diabetes (JSN and JFK, manuscript in preparation). We have also observed the deposition of GlcNAc reactive IgM on glomerular membranes following kidney ischemia reperfusion injury models (unpublished observations, JSN, JFK, and Dr. Alex Szalai, University of Alabama at Birmingham). Collectively, these observations strongly suggest that natural antibodies specific for truncated glycan products may function analogously to phospholipid- and oxidized lipid-specific nAbs, reducing the immunogenicity of apoptotic cell-associated determinants, thereby attenuating inflammation driven by innate-recognition of aberrant post-translational modification of autologous antigens.
Despite evidence that alterations in glycosylation profiles contribute to recognition of apoptotic cells, it is still unclear how these structures are generated. In cases of apoptosis-associated lipid neodeterminants, enzyme-mediated relocalization of lipid-membrane molecules drives apoptotic cell recognition (22). Whether truncated glycans generated on apoptotic cells, such as GlcNAc-terminating glycans, are derived through glycosidase-dependent degradation of mature glycan structures, or from premature mobilization of ER and Golgi-derived incomplete glycan structures is not clear (52). Although glycosylase-activity is high in lysosomal compartments and could potentially expose cryptic glycan epitopes during autophagy, Rho GTPase activity during apoptosis may relocate endoplasmic reticulum membrane fragments to the plasma membrane, facilitating exposure of immature glycophospholipids (56). Regardless of the mechanism, exposure of truncated glycans on apoptotic cells is a regulated process, and antibody reactivity with cryptic glycan epitopes clearly represent a form of natural autoimmunity that is not currently understood.
In addition to directing phagocyte recognition of pathogen- and apoptotic cell-associated glycan structures, changes in glycosylation patterns mediate several other important immunological processes (52, 55, 57). During ischemia reperfusion induced vascular injury, hypoxia-driven changes in endothelial cell glycosylation drives innate complement activation through mannose-binding lectin (58). Furthermore, oncodevelopmental glycan antigens have been described in some carcinomas, in which altered glycosylation profiles correlate with tumor cell growth and differentiation (59, 60). Circulating glycan-reactive nAbs levels influence the outcome of these processes, as suggested by recent reports low IgM levels towards advanced-glycation end-products correlated with a high-risk of cardiovascular events in non-diabetic patients (61). Further support for the physiological relevance of glycan-reactive antibodies comes from the observation that certain glycan-reactive antibodies participate in several autoimmune disease settings. Antibodies reactive with high-mannose glycan structures are found in the cerebrospinal fluid of patients with multiple sclerosis (MS) as well as in mice with experimental autoimmune encephalomyelitis, and correlate with significant protection from disease (62). Alternatively, Glucoseα-1,4-Glcα-reactive antibodies are associated with relapse following MS diagnosis (63). Antibodies reactive with chitibiose (GlcNAcβ-1,4-GlcNAcβ ) and laminaribiose (Glcβ-1,3-Glcβ) are observed in Crohn’s Disease (CD) patients, correlate with disease severity, and differentiate CD from ulcerative colitis (64, 65). Although the physiological influences that contribute to the production of these antibodies remains unclear, these observations provide evidence that implicate carbohydrate-reactive antibodies in the progression of several autoimmune diseases. These observations also suggest that glycan-sensing is central to effective host-protective immuno-surveillance, tolerance, and tissue homeostasis, including tumor surveillance.
B-1 cells: the source of natural antibodies
This section summarizes and discusses the development and function of B-1 B cells, the population long-associated with nAb secretion (66). B-1 B cell development begins in the fetal liver with yolk sack-derived B cell progenitors. B-1 B cells constitute the major emerging B cell population in neonates, but become less represented in the spleen relative to the MZ and Fo B cells in the adult (67). Mature B-1 B cells are found in the adult spleen, as well as peritoneal and pleural cavities, where their populations are maintained by the self-renewal(1). Unlike naïve FO B cells, B-1 B cells exist in a so-called semi-activated state, displaying cell surface marker expression (68, 69), gene expression profiles (70), and BCR complex distribution (71) characteristic of B cells that have experienced prior antigen stimulation. These observations have led some to refer to the B-1 B cell reservoir as a compartment of B cell memory. Accordingly, B-1 B cells display robust proliferative responses, and can rapidly differentiate into antibody secreting cells following BCR-ligation (2). B-1 B cells are responsible for producing most serum IgM, including natural IgM, and they contribute to T-independent class switched antibody responses to produce IgG (predominantly IgG3 in mice), and IgA antibodies (72, 73). The significance of B-1 B cell-derived IgA is discussed later in this review.
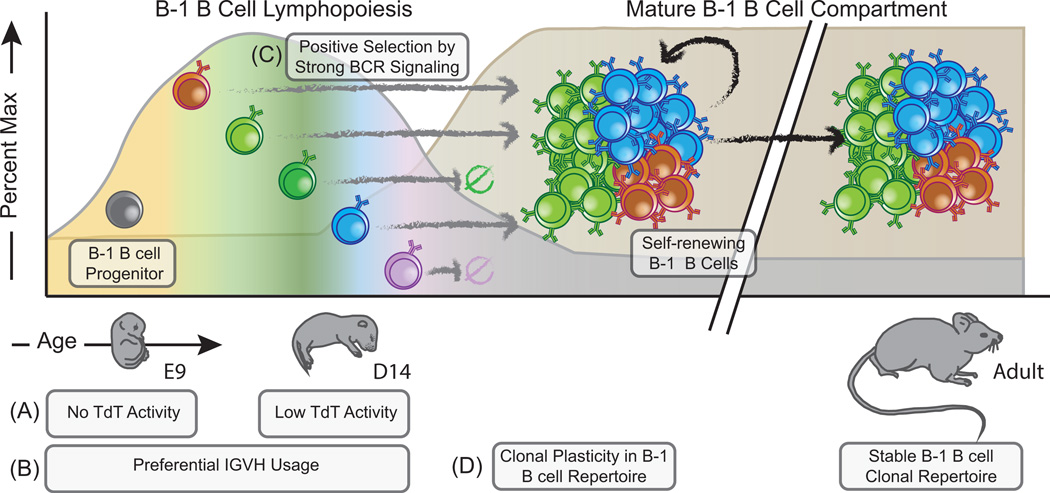
It is clear that B-1 B cells exhibit restricted antigenic reactivity in their BCR repertoire, which includes multi/self-reactive antibodies of relatively low affinity. Multiple genetic mechanisms unique to B cells arising during fetal and neonatal life contribute to the restricted pre-immune B cell repertoire observed in the B-1 B cell compartment (74). The absence of terminal deoxynucleotidyl transferase (TdT) expression in fetal B cell progenitors (Figure 4A) (75) is one of the most well-understood mechanisms governing BCR repertoire establishment during B cell ontogeny. Decreased junctional diversity, as a consequence of the absence of TdT-mediated N-nucleotide addition and exonuclease activity, exerts multiple effects that restrict the expressed B cell repertoire. Germline IgH genes have limited pairing potential with the V-lambda 5 surrogate light chain and rearranged light chain gene products, as well as biased D reading-frame usage due to limitations in homology-directed end-joining during V(D)J recombination (75–78). Fetal and neonatal B-1 B cell clonal development also demonstrates preferential usage of more D-proximal VH genes, notably VH81X, which are rarely expressed in functional rearrangements of B cells found in adulthood (79, 80). Changes in genetic restrictions on V(D)J recombination manifest as the temporal emergence and representation of distinct B cell clonotypes in the innate-like B cell pool (Figure 4B). Yet further restriction of the expressed Ig repertoire occurs via the preference for strong BCR signaling during pre-B cell selection in early B-1 B cell lymphopoiesis, which promotes an enrichment of autoreactive BCRs in innate-like B cell compartments by selection events occurring in the context of autologous antigen (Figure 4C) (81–83). Thus, restrictions on combinatorial diversity during early B-1 B cell development promote characteristic Ig-gene profiles and autoreactive B cell clonotypes.
Figure 4. The population of the B-1 B cell repertoire is highly temporal, and is shaped by genetic restrictions on B cell receptor recombination and by positive-selection mechanisms.
B-1 B cell committed progenitors have been identified in embryonic mice as early as day 9. There is a clear hierarchy regarding the emergence of various B cell clonal-specificities shaped by both the absence of terminal deoxynucleotidyl transferase activity in fetal B cells (A) and preferential IGHV gene usage (B). B-1 B cell lymphopoiesis continues through neonatal and perinatal development, during which period TdT is active at low levels. Together, these mechanisms result in a dynamic appearance of characteristic clonal BCR rearrangements (indicated by distinct colors). (C) Following successful rearrangement of clonal B cell receptors, B-1 B cells undergo positive selection in order to become mature B-1 B cells. These selection events occur in the context of autologous antigens, giving rise to autoreactive, CD5+ B-1a B cells, or through exogenous antigen, giving rise to CD5- B-1b B cells. Once mature, these cells populate the peritoneal and pleural cavities, where they are maintained through self-renewal. (D) Clonal specificities represented in the B-1 B cell pool are highly stable throughout adult life. Thus, the neonatal and perinatal periods represent a critical time of B-1 cell repertoire plasticity, wherein environmental antigens significantly influence the clonal composition of this population. The outcomes of these early developmental and environmental influences on the B-1 B cell repertoire have significant impacts on immunity and the propensity to develop aberrant allergic or autoimmune phenomenon during adult life.
In the case of well-characterized anti-PC antibodies, forced expression of a TdT transgene results in production of non-canonical anti-PC antibodies, which are not protective against virulent pneumococcal infection in adult animals (84). Importantly, we and others observe evidence of low levels of N-nucleotide additions in the BCR products of perinatal B cell clones (Shown in Figure 4B), suggesting that the genetic control of V(D)J restriction of early B cell lymphopoiesis may be diminishing during this time (JSN and RGK, unpublished observation) (L.A. Herzenberg, Communication from Merinoff B-1 B Cell World Congress). Despite some B-1 B cell production in adult life (85, 86), the combinatorial restrictions apparent during neonatal B cell lymphopoiesis do not occur in adult bone marrow (BM), and for this reason, adult and perinatal B-1 B cell lymphopoeitic potential are qualitatively distinct. Importantly, we have found that the innate-like B cell repertoire in adulthood is largely determined by early immune system development, during which time there is increased clonal plasticity in the B cell compartment (Figure 4D) (5). Specificities of the B-1 B cell repertoire are therefore shaped temporally by changing restrictions on BCR recombination leading to the emergence of specific clonotypes.
There are two generally accepted models of mouse B-1 B cell development: the lineage-hypothesis and the ligand-dependent model. It is clear that neither model fully describes mechanisms involved in B-1 B cell development, and there is evidence supporting both models of developmental origin. The lineage hypothesis suggests that B cell subsets are derived differentially based on the distinct B-cell precursors that specifically fill their respective niches. Supporting this model is the well-documented propensity of mouse fetal B cell precursors to generate B-1 B cells (66), with clear differences in costimulatory molecule expression during ontogenetic development of B-1 and B-2 B cells (85). More recently, bona fide AA4.1(+)CD19(+)B220(low-neg) B cell precursors that selectively reconstitute B-1 and Marginal Zone B cells were identified at embryonic day 9 (87, 88), and B-1 B cell specific transcriptional programs were described (89). Collectively, these observations suggest that mouse B-1 B cells are derived from a committed progenitor. Alternatively, the ligand-dependent model suggests that the B-1 B cell subset phenotype results from the context of antigen-dependent BCR engagement differentially experienced by a single B cell progenitor. This model is supported by numerous observations that BCR signaling strength directly influences acquisition of B-1 and Marginal Zone B cell phenotypes (90–93). In this scenario, B-1 B cell selection is a competitive process involving immunogenic and autologous forms of antigen that mediate qualitatively different signals during BCR selection, and the relative contributions of these antigens to clonal development are determined by both the timing of antigen exposure and relative BCR-derived signal intensity (94). BCR ligands bearing autologous glycan profiles can increase the threshold of BCR signaling required for NFkB activation through engagement of Immunoreceptor Tyrosine-based Inhibition Motif- (ITIM)-containing Sialic-acid binding lectin of the Immunoglobulin-superfamily–G (Siglec-G) (95). These signals can drastically affect the ability of innate-like B cell clones to undergo antigen-mediated positive selection , which illustrates the complex nature of how endogenous antigens influence formation of the natural antibody repertoire.
Expression of CD5, another ITIM-containing costimulatory molecule, correlates with strong autoreactive BCR signaling during selection (96), and segregates the peritoneal and pleural B-1 B cell populations into the CD5+ B-1a and the CD5− B-1b B cell compartments. B-1a B cells first emerge during fetal development, whereas the B-1b B cell compartment is seeded later during the neonatal period. Both subsets contribute significantly to pathogen-induced T-independent antibody responses, and are capable of robust proliferation and plasma cell differentiation in the generation of host immunity (10). In response to some pathogens, such as S. pneuominae, both B-1a and B-1b B cells respond to infection but perform distinct, non-redundant roles at different phases of the immune response (97).
B-1 B cell activation results in IgM antibodies that persist for long periods of time relative to the IgM derived from T lymphocyte-dependent germinal center antibody responses (98–100). A partial explanation for the maintenance of these long-term antibody responses is the retention and persistence of antigen in lymphoid tissues (101). Importantly, B-1 B cell responses result in clonal enrichment of cells within the self-renewing B cell pool, that display characteristics of immunological memory, including robust clonal proliferation and rapid antibody-secreting cell differentiation upon secondary antigen exposure (98, 99, 102, 103) (JSN and RGK, unpublished observations). The peritoneal and pleural B-1 B cell compartments therefore appear to serve as the principal reservoirs for immunological memory that provides long-lasting neutralizing immunity to T-independent antigens.
The B-1 B cell compartment contains precursors of natural antibody-secreting cells that, upon antigenic stimulation, undergo a plasma cell differentiation program that is distinct from that of germinal center-derived plasma cells. B-1 B cells migrate to the spleen where they differentiate into natural antibody secreting cells (ASCs), and are maintained by the cytokine interleukin-5 (73, 100, 104). More recently, Reynolds et al. described a specific population of BM ASCs that were the primary source of circulating IgM antibodies (105). Although it is unclear if the similarity in surface phenotype of these cells (mIgM+CD138+ B220lo/−FSChi) to that of plasmablasts described by other groups (106) is indicative of a short-lived nature of these ASCs. It is possible that long-term maintenance of serum nAbs requires continuous replenishment of ASC niches from non-terminally differentiated peritoneal cavity B-1 B cells.
Collectively, these observations demonstrate that the emerging B cell repertoire is influenced by genetic constraints that contribute significantly to the formation of the B-1 B cell compartments. However, B cell clonal selection and expansion resulting from antigen exposure also contribute to repertoire development. The clonal repertoire exhibits considerable plasticity during neonatal development, and changes induced by immunization in this period permanently alter clonal representation in adult B cell responses (5). In the next section, we examine how B-1 B cell programmed development interplays with antigenic exposure, leading to differential clonal representation in the adult B-1 B cell compartment.
Neonatal development of polysaccharide-specific B cells and implications for the adult B cell repertoire
During the neonatal period, because B-1 B cell and plasma cell niches are not yet filled, there is significantly lower inter-clonal competition relative to later stages in life. We have reported that neonatal and perinatal immunization results in altered clonal expansion of antigen-reactive B cells that persist in the adult repertoire (5, 107). These phenomena are illustrated by the PC-specific natural antibodies bearing the TEPC15 (T15)-idiotype (Id), which are highly correlated with protection from S. pneumoniae infection and the suppression of both autoimmunity and allergies (5, 31). Canonical T15-antibodies are characterized by utilization of VHS107 as well as Vκ22 light chain gene segments, and are clonally expanded upon immunization with Streptococcus pneumoniae. Neonatal exposure to S. pneumoniae, however, results in preferential expansion of clones utilizing alternative Vκ24 and Vκ8 light chains (5). These manipulations of the PC-specific B cell pool, driven by neonatal antigen exposure, result in a loss of protective antibody responses during virulent S. pneumoniae infection in adulthood; however, these M167-Id bearing PC-specific B cell clonotypes suppress the development of house-dust mite-induced allergies (31). Therefore, evolutionary conservation of Ig-alleles within the BCR locus, ontogenetic constraints, and the availability of exogenous antigen during perinatal development together influence clonal B-1 B cell representation in the adult repertoire.
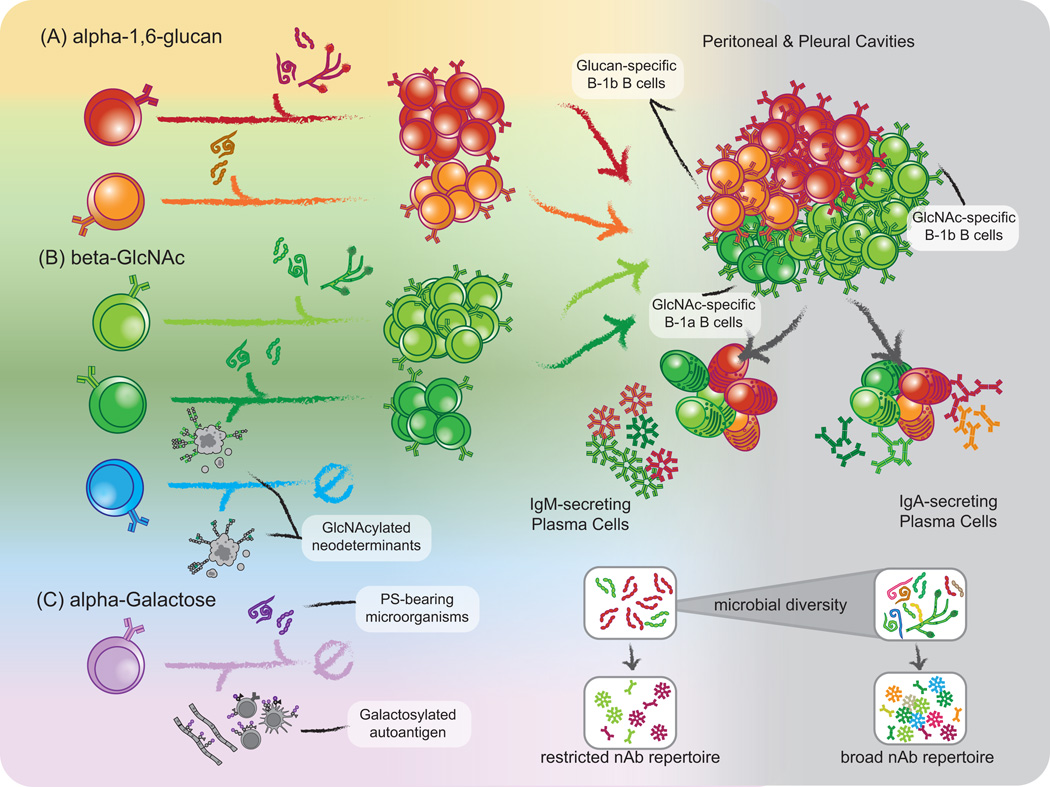
Although the antigenic factors directing the development and composition of the natural antibody repertoire remain poorly understood, it is clear that perinatal antigen experience can affect the magnitude of clonal B cell responses. Long-held observations show that neonates display poor antibody responses to polysaccharide-immunization, and it is clear that early neonatal B cell responses differ quantitatively from those of adult mice. Although perinatal antigen exposure does not elicit robust antibody responses in neonates, we and others have observed that early intervention with antigen alters the frequency of antigen specific clonotypes, which result the production of qualitatively different antibodies of similar, if not identical specificity after appropriate antigen immunization of the adult (108). Thus, antigen experience during the neonatal period is a critical factor in determining the specificities represented within the B-1 B cell compartment. In the following sub-sections, we review three examples of B-1b B cell-derived polysaccharide-specific natural antibodies that illustrate how glycan-specific natural antibody production involves the integration of BCR signals derived from both autologous antigens and pathogen-associated exogenous antigens: i) α-1,3-dextran, ii) β-1,4- and −1,6-GlcNAc, and iii) α-1,3-galactose. The source and relative abundance of these moieties on antigens are varied, resulting in dramatic differences in the development and functions of B cells reactive with these epitopes. Accordingly, comparisons of these three well-described systems illustrate the dichotomous effects of antigen availability and inter-clonal completion that influence B-1 B cell development, and ultimately determine the specificities represented within the glycan-reactive natural IgM repertoire.
Alpha-1,3-glucan-reactive B cell Development
α-1,3-glucan polysaccharide, (including the branching PS-structures on the α-1,6-glucan backbones of dextran) contains glycan epitopes that are widely expressed on bacteria and fungi, but are absent from the mammalian glycan repertoire. Therefore any antigen experience of α-1,3-glucan-specific B cells, which typically display a B-1b or MZ B cell phenotype, is derived from exogenous sources (Figure 5A) (98). T-lymphocyte independent antibody responses to α-1–3-dextran can be elicited by immunization with Enterobacter cloacae, or purified α-1,3-Dex. Furthermore, α-1,3-Dex-reactive antibodies bind members of the Enterobacteriaceae family isolated from the normal gut microflora of mice, suggesting that members of the commensal flora may provide a glycan source that influences the development and functions of B cells with this reactivity (109). Supporting this concept, the magnitude of Dex-reactive Ab responses following immunization with Enterobacter is significantly reduced in germ-free mice relative to conventionally reared counterparts, suggesting there is a lower frequency of Dex-specific ASC precursors in the absence of normal flora (109).
Figure 5. The interplay of endogenous and exogenous antigen signaling on innate-like B cell repertoire formation.
Exogenous antigen contributes significantly to clonal representation in the mature B-1 B cell pool, and the complexity of these events is demonstrated through analysis of α-1,6-glucan, β-GlcNAc, and α-1,3-Gal B cell specificities. (A) α-1,3-glucan structures are absent from the mammalian glycome and Dex-specific B cells develop in the absence of stimulation by endogenous glucan epitopes. There are two canonical clonotypes within the Dex-reactive B cell pool, M104E (orange) and J558 (red), the latter of which is more highly represented in the adult repertoire. Glucan-specific B cells possess mainly a B-1b and MZ B cell phenotype, suggesting that exogenous antigen promotes selection of this clonotype into the B cell pool. In support of this interpretation, the numbers of Dex-specific B cell precursors are significantly reduced in mice raised in germ-free conditions, suggesting exogenous antigen is solely responsible for selection or maintenance of cells bearing this specificity. (B) Contrary to Dex-specific B cells, GlcNAc-specific B cells develop in the context of several forms of GlcNAcylated autoantigen, although these cryptic epitopes may provide a limiting developmental signaling to GlcNAc-reactive B cell precursors. In conventionally housed mice, GlcNAc specific cells are predominantly represented in the B-1b (lightgreen) compartment, but are also observed within the B-1a (darkgreen) population. In germ-free conditions, there is a stark decrease in the numbers of GlcNAc-specific cells in both B-1 B cell compartments (blue). Given that the B-1a phenotype is generally associated with autoreactivity, these observations suggest GlcNAc-specific B-1b B cells depend solely on exogenous antigen for their development, whereas exogenous antigen may rescue GlcNAc specific B-1a clonotypes from negative selection following engagement of autologous glycans. (C) Lastly, α-1,3-galactose epitopes, generated by α-Galactosyl Transferase (GalT) are common terminating moieties of N-linked and O-linked glycan chains in mice and drive negative selection of Gal-reactive B cells. Thus, Gal-reactivity is absent from the adult repertoire. In GalT-deficient mice, however, α-1,3-galactose-specific B cells develop, adopt a B-1b B cell phenotype, and are clonally expanded through interactions with commensal microbiota-derived antigens. These examples of three independent B-1b B cell glycan specificities illustrate how integration of signals derived from both endogenous and exogenous forms of homologous carbohydrate epitopes contribute to selection of the anti-glycan repertoire. As the B-1 B cell pool represents precursors of both natural IgM and secretory IgA producing plasma cells, the representation of certain clonal specificities within these expressed natural antibody and mucosal secretory IgA repertoires relies on exogenous antigen.
α-1,3-glucan-specific antibodies are highly restricted clonally, being encoded by a single VH gene present in the IgMA (IgH6a) locus, whose protein product becomes paired with a lambda light chain. These antibodies are absent in certain mouse stains, including C57BL/6 which possess the IgMB locus. α-1,3-glucan-specific BCR formation also requires N-nucleotide addition to encode an aspartic acid residue (D99) at the V-D junction, and as such, TdT-deficient IgH6a (IgMA) animals respond poorly to immunization with α-1,3-glucan-bearing microorganisms (110, 111). Two major clonotypes within the α-1,3-glucan-reactive B cell populations are represented in the adult B cell repertoire. The dominant J558 (Figure 5A, red) and the minor M104E (Figure 5A, orange) clonotypes utilize Ig heavy chains that differ by only two amino acids (112). Similar to the scenario in PC-specific clonal B cell responses, the ratio of the minor M104E clonotype to dominant J558 clonotype frequency is inverted in neonatal B cell precursors compared to that observed in the adult B cell repertoire. This observation may indicate the preferential expansion of precursors bearing J558 idiotypes by commensal-derived antigens.
Thus, in the case of the anti-α-1,3-glucan-repitoire, innate-like B cells are expanded solely based on exogenous antigen, likely derived from the commensal flora, because autologous α-1,3-glucan-structures are unavailable to influence innate-like B cell development.
N-acetyl-D-Glucosamine-reactive B cell development
As discussed above, N-acetyl-D-glucosamine is a ubiquitous epitope found on many polysaccharide antigens in Gram-positive and Gram-negative bacteria, fungi, protozoan pathogens, and parasitic worm species (20). Furthermore, GlcNAcylation occurs in mammalian cells in a variety of protein post translational modifications, including the core structure of N-linked glycans, complex O-linked glycans, and regulatory monomeric O-GlcNAc modifications (113). However, these endogenous GlcNAc residues are frequently cryptic and their accessibility to B cells and antibodies is unclear. Despite the potential for pathogenic autoreactivity, GlcNAc-reactive B cells and anti-GlcNAc antibodies are common in mammals, and are functionally responsive. Group A Streptococcal infections induce GlcNAc-reactive antibodies that target the Group A Carbohydrate cell wall antigen of GAS. GlcNAc-reactive B cells therefore have the potential to respond to various GlcNAc-bearing autologous antigens in addition to pathogen-associated forms of GlcNAc (Figure 5B).
Early studies in mice suggest that GlcNAc-specific antibody clonotypes are highly restricted in their IGHV gene usage, giving rise to a pauciclonal antibody repertoire (114, 115). GlcNAc-specific antibodies in humans are also restricted, and idiotypes of GlcNAc-specific Abs appear to be highly conserved in the human population (49, 116). Interestingly, certain GlcNAc-reactive Ab-associated idiotypes in humans demonstrate antibody isotype-restriction (117), which may be indicative of differing roles of variable fine-specificities within the GlcNAc-specific B cell compartment.
We find that individual GlcNAc-specific B cell clonotypes clearly exhibit sensitivity to exogenous antigen. We observed that two major GlcNAc-specific B-1 B cell clonotypes can be found in mice: the first utilize VHJ606-encoded receptors, are frequent in the adult repertoire, and have been extensively characterized (Figure 5B, light green) (115), the second is a minor clone which utilize VHS107 heavy chains and has not been described previously (Figure 5B, dark green). After neonatal immunization of C57BL/6 mice with heat-killed, pepsin-treated preparations of Group A Streptococcus, we observe significant alterations in the relative frequencies of these two dominant clonotypes, with early immunization expanding the VHS107-utilizing clones. Like the situations in PC- and α-1,3-glucan-specific B cell clonotypes, the relative abundance of these two distinct clonotypes is reversed in the neonatal pool of GlcNAc-specific B cells, and perinatal immunization with GAS drives increased representation of a typically minor clonotype in the adult repertoire.
As illustrated in Figure 3, antibodies elicited by GAS immunization react with cryptic GlcNAcβ-1,4- and GlcNAcβ-1,6- epitopes within the core of N-linked glycans. However the accessibility of these antigens to B cell precursors is uncertain, and cross-reactivity for various forms of endogenous GlcNAcylated antigens is likely highly dependent on subtle differences in fine-specificity of clonal BCRs. Most GlcNAc-specific B cells possess B-1b and MZ B cell phenotypes; however CD5+ B-1a GlcNAc-binding B cells are also observed. Intriguingly, these clonotypes are absent from the peritoneal B cell pool of germ-free mice (J.S.N., R.G.K., and J.F.K., unpublished observation), suggesting that autologous GlcNAc-containing glycans are insufficient for the maintenance of this specificity within these B cell compartments. Although the accessibility and relative density of GlcNAc epitopes on macromolecules are likely major factors in this process, it appears that clonal representation of autoreactive GlcNAc-specific B cells results from stimulation by similar forms of exogenous antigens, and the availability of these antigens during perinatal development may rescue these clonotypes from negative selection by GlcNAc-containing self-antigens (Figure 5B, blue). GlcNAc-specific B cell developmental requirements are heterogeneous, as this reactivity is comprised of clones with differing fine-specificities for autologous GlcNAcylated structures. Although many of these clonoypes recognize autologous GlcNAc, environmentally derived antigen appears to be required for their representation in the adult B cell pool.
alpha-1,3-Galactose-specific B cell development
α-1,3-Galactose (α-1,3-Gal) specific antibodies were originally identified as mediators of hyper-acute graft rejection following pig-to-human xeno-organ transplant, due to the differential presence of this glycan structure on porcine tissues (118). In the context of natural antibodies, α-1,3-Gal epitopes are an interesting specificity. In contrast to other species, the absence of the α-1,3-galatosyltransferase (GalT) enzyme in old world apes and in humans permits the development of antibodies specific for α-1,3-Gal. Similarly to α-1,3-Glucan-specific B cells, Gal-specific B cells in old world apes and humans develop in the absence of autologous α-1,3-Gal epitopes (119). In mice, GalT activity is intact and anti-Gal B cells and antibodies are completely absent. Galactose epitopes are more commonly incorporated as terminal moieties of mature glycan chains than are GlcNAc epitopes, and may more effectively drive negative selection of Gal-reactive BCRs during development (Figure 5C).
Interestingly, alpha-1,3-Gal-specific antibodies are spontaneously represented in peripheral antibody repertoire of mice following the genetic ablation of the GalT gene. Similarly to α-1,3-Glucan-reactive B cells, Gal-reactive B cells engage antigens derived from the commensal microbiota, are further expanded by colonization with antigen-bearing bacteria (E. coli (O86:B7)), and display a CD5−CD43+ B-1b B cell phenotype (120). These observations suggest that despite the existence of bacteria-associated Gal-antigens within the microbiota, the availability of autologous Gal-antigen to mediate negative selection of Gal-specific B cells is too great. Indeed, even under knock-in expression of α-1,3-Gal-specific IgM heavy and light chain transgenes derived from the M86 hybridoma, Gal-reactive B cells undergo receptor editing in mice when GalT activity is intact (121). These observations provide a scenario in which the relative accessibility of autologous and exogenous glycan epitopes exert counteracting pressures during the selective processes governing innate-like B cell production.
Anti-Gal antibodies are the most abundant of natural immunoglobulin in humans and display IGHV3 gene restriction (119). Evidence for affinity maturation by analysis of somatic hypermutation distribution in α-1,3-Gal-specific antibody sequences suggest that these clonal rearrangements have undergone antigen selection. It is likely these cells have affinity maturated against epitopes on exogenous antigens including, but not limited, to bacterial polysaccharides derived from the intestinal microbiota (122). Similarly to other polysaccharide antigens recognized by the B-1 B cell repertoire, αGal antigens are represented on various mammalian pathogens, including Plasmodium falciparum. Remarkably, colonization of GalT-deficient mice with E. coli O86:B7 led to induction of B-1b B cell derived nAbs that provided sterilizing protection against this Plasmodium falciparum, and could potentially reduce malaria transmission in humans (123).
The role of glycan antigens in the development of the natural antibody repertoire
It is commonly stated that natural antibodies are those that develop without influence by external antigenic stimulation, and in some situations, as is the case of phosphorycholine-reactive B cell clonotypes, endogenous antigen sources may suffice to drive selection of these innate-like B cells (10, 124, 125). However, in the case of glycan-specific B cells, including those discussed above, consistent observations show a dependence on exogenous and pathogen-associated antigens for their development. Antibodies derived from some of these PS-specific B cell clonotypes do however recognize cryptic epitopes contained within autologous glycans. Thus, the generalization often made that the natural antibody repertoire develops independently of exogenous antigen is not universal for all nAb specificities, and further research focusing on the factors contributing to the development and the composition of the nAb repertoire is warranted.
It is clear that the nAb-secreting plasma cell pool size is tightly controlled, most likely by intrinsic homeostatic mechanisms (126), because mice reared in the absence of antigenic stimulation (i.e., under germ-free conditions receiving an chemically-defined diet) demonstrate normal levels of IgM antibodies relative to conventionally-raised counterparts, whereas other isotypes such as IgG and IgA are greatly decreased (127, 128). However, numerous experimental observations demonstrate that the timing of exogenous antigen exposure during neonatal and perinatal B cell lymphopoiesis can drastically alter the clonal representation of antigen-specific B cells in the adult mouse repertoire (3, 31, 108). Together, these observations suggest that early exposure to exogenous antigens is critical for driving B cell clonal representation of these specificities in the limited nAb-secreting plasma cell niche.
Consistent with this model, natural antibodies recognizing carbohydrates are not detectable during the first weeks of life. Additionally, in early studies G.F. Springer found that isoantibodies against heterologous blood group antigens could be elicited in humans following oral or intranasal immunization with Escherichia coli O(86), bearing polysaccharide antigens orthologous to human blood group B antigen. His observations led him to propose the “bacterial paradigm”, suggesting that carbohydrate-specific nAbs are a product of immune stimulation by bacterial antigens of gastrointestinal bacteria (129). Springer’s early studies demonstrated that microbiota-derived polysaccharides are key in shaping the innate-like B cell repertoire, however later studies examining the content of the IgM repertoire in mice raised under germ-free conditions and fed chemically-defined diets challenged the role of exogenous antigen in establishing the IgM repertoire (130, 131). In recent, more global analyses, Bos et al. clarified that certain specificities represented in the natural antibody repertoire are highly influenced by microbial colonization (132). Additional reports of variation in B cell clonal precursor frequencies with colonization status further suggest that antigenic stimulation expands individual clones during repertoire development (80, 109). In the coming subsections, we investigate the interactions of B-1 B cells with the intestinal microbiota, and how these interactions affect nAb repertoire development.
Glycans in host-microbiota symbiosis
Many pathogens have evolved strategies to evade immune detection through manipulation of their glycan signatures. For example, Bifidobacterium spp. and Bacteroides species scavenge and incorporate host-derived carbohydrate residues into their capsular polysaccharides and glycoproteins (133). Bacteroides require O-glycosylation pathways for colonization in the presence of a competitive microbiota, and maintain highly heterogeneous polysaccharide profiles by phase-variation of glycosyltransferase genes (134). The host also responds to altered intestinal homeostasis with glycosylation profile changes on epithelial cells. Type three innate lymphoid cells, by producing interleukin-22, control fucosylation of host epithelial cells and secretion of fucoslyated mucin, contributing to the formation of an antimicrobial platform and a cohabitation niche for the establishment of normal microflora (135). Elucidation of these pathways demonstrate that polysaccharide epitopes are central to immune system interactions with the intestinal microbiota; however, the mechanisms involved in polysaccharide-reactive B cell maintenance of immune homeostasis are poorly understood.
Interactions of innate-like B cell repertoire and commensal flora
As mentioned above, innate-like B cells can perform class-switch recombination in extra follicular, T-independent responses (136, 137). B-1 B cells display a higher propensity than FO B cells to generate IgA-secreting plasma cells, and have long been considered a likely reservoir of precursors for intestinal IgA plasma cells (72, 138). Although the direct contributions of B-1 B cells to IgA in mucosal secretions has been controversial (139, 140), recent studies provide strong evidence of B-1b B cell involvement in intestinal T-independent IgA antibody responses that, like natural IgM-secreting B-1 B cells, are IL-5-dependent (141, 142). Unlike T-dependent IgA responses that target pathogenic bacteria present or introduced into the microflora, B-1b B cell-derived IgA is important in the homeostatic maintenance of commensal organisms (143). Secretory IgA (sIgA) facilitates colonization with certain microbes, inducing tolerogenic DC phenotypes and, in some cases, supporting biofilm formation by commensal bacteria (144, 145). In this capacity, sIgA sensing of microbial epitopes is crucial in maintaining host-commensal symbiosis. Although significant portions of the intestinal microbiota were once believed to be opsonized by sIgA, more recent reports suggest that this fraction of the intestinal microbiota contains low-frequency bacteria, not detectable in typical rDNA-sequencing analyses (146). Interestingly, it has been suggested that these bacteria represent a core microbiome of sorts, composed of highly stable taxa widely conserved in healthy individuals. Understanding what antigenic specificities direct these antibody-bacteria interactions could yield significant insight into the contributions of specific B-1 B cell clones to sIgA production and maintenance of a healthy microbiota .
As discussed above, antigen experience during neonatal and perinatal development is essential for determining the clonal specificities represented in the adult B cell repertoire. Importantly, these developmental periods are characterized by increased intestinal permeability, resulting in high systemic exposure to intestinal microbiota-derived antigens. These antigens represent potential substrates for BCR-selection processes during innate-like B cell development, and may translocate to specialized microenvironments in the spleen to mediate ligand-dependent positive selection of B-1 B cell precursors (147, 148). Information regarding the recruitment of B-1 B cell clonal specificities into the IgA ASC pool is limited, however considering the temporal nature of the development of clonal-specificities within this B cell compartment, it is clear how diversity in the microbiota early in life could influence the specificities of secreted IgA. The observed enrichment of PS-reactive clonotypes within B-1b B cells and the growing appreciation of the involvement of glycan antigens in host-commensal interactions suggest that colonization by certain microbes may differentially recruit antigen-specificities into the B-1 B cell compartment. This would in-turn influence the expressed IgA repertoire thereby facilitating the stable colonization of these commensal bacteria. This interplay is likely ontogenetically restricted, because both intestinal permeability and neonatal B-1 B cell development normally occur only temporally and early in life.
In mice, the first appearance of IgM+ and IgA+ B cells and plasma cells at mucosal sites occurs rapidly, within a few hours after birth (149). A similar progression has been noted in humans, where plasma cells are first observable in gut and later in bronchi in fetal stages (150). The IgA repertoire in early life is dominated by low numbers of expanded clonotypes with few mutations (151, 152). These early appearing clones have been proposed to serve as founder B cells, and to drive formation of a highly stable IgA repertoire wherein the global specificities resist alteration following antibiotic treatment, bacterial infection, and plasma cell depletion (153, 154). Antigenic specificities represented in the sIgA pool are stable over time, and gradually accumulate mutations with age, appearing to be fine-tuned by the antigen content of the intestinal microbiota (154). These observations suggest that IgA precursor cells are derived from a memory B cell compartment and participate in microbiota-induced immune responses localized to gut-associated lymphoid tissues (154). In activation-induced cytidine deamidase-deficient animals that cannot switch antibody classes, IgM compensated for loss of IgA in mucosal secretions, targeting the same commensal bacteria (141), suggesting these IgA-secreting plasma cells share a consistent origin with IgM-secreting B-1 B cells. Despite the growing appreciation of innate-like B cells in maintaining the intestinal microbiota, how microbiota-derived antigens influence the natural antibody repertoire remains poorly understood.
Implications for mucosal B-1 B cell and microbiota interactions in the developing nAb repertoire
It remains unclear to what extent exogenous antigens, including those derived from the commensal microbiota affect the antigenic specificities represented in the natural antibody-producing B cell pool. As discussed above, several known B-1a B cell clonotypes develop normally in conditions of restricted exogenous antigen (gnotobiotic conditions) (155); however, there are clear differences in IGHV-gene utilization in the expressed B cell repertoire of germ-free mice (80), and overwhelming evidence reviewed above demonstrate that the neonatal and perinatal antigen experience can dramatically alter the clonal-specificities represented in the adult B cell repertoire (5). Considering that the B-1 B cell compartment contributes to both natural IgM- and secretory IgA-producing antibody secreting cells, it is likely that these antibodies, sourced from the same compartment, are linked by their temporal development through antigenic stimulation at mucosal sites, lending further credence to the notion that exogenous antigen may participate in the peripheral selection of innate-like B cell progenitors.
Immunoglobulin sequences derived from IgA+ B-1 B cells, like those derived from IgM+ B-1 B cells, are largely germ-line, possess few mutations, and show little, if any, sign of affinity maturation (156). Shared specificities of B-1 B cell derived antibodies, expressed as different isotypes, may function to suppress responses towards potentially inflammatory analogous-epitopes present on aberrant self-antigens while also contributing to the maintenance of healthy symbiotic bacterial microflora.
Numerous reports show that commensal microbiota-derived antigen is important in early clonal selection events of B cell precursors. Some reports have suggested that these antigens may be translocated to the perinatal spleen, which may serve as a location for pre-B cell selection on exogenous antigen (147). The mechanisms driving these pathways remain poorly understood, but evidence suggests that this phenomenon may occur in the splenic Marginal Zone (148). Furthermore, others have described B cell selection events occurring directly in the gut: immature B cells maintain expression of recombination-activating genes after migrating to the intestinal lamina propria, where receptor-editing processes occur in the context of microbial antigens (157). Despite limited evidence directly linking B-1 B cell immunoregulatory function to microbial diversity, diversity in the intestinal microflora is essential for producing regulatory B cells that can support tissue engraftment (158). Graft rejection was accelerated in animals raised under gnotobiotic conditions or when microbial diversity was abrogated by antibiotic treatment of mice housed in conventional conditions. These early reports may implicate members of the commensal flora in antigen-driven clonal expansion of innate-like B cells with regulatory functions. Further understanding the interplay between microbiota-derived antigens, B cell clonal selection, and repertoire development is central to the future design of nAb-based therapies for allergic and autoimmune diseases.
From genes to therapies- the future of nAb-based diagnostics and therapeutics
As we have reviewed, natural IgM is important for host-defense and maintaining immunological homeostasis. Our recent studies validate that this antibody class represents a promising candidate for the development of both diagnostic tools and therapies for allergic and autoimmune diseases (5).
PC-reactive B cell clonotypes, boosted via immunization with PC-bearing S. pneuomiae provide significant atheroprotective functions to mice fed a high-fat diet (27). Following these studies, phosphorylcholine-reactive antibodies have proven useful for diagnosing patients at risk for cardiovascular diseases such as atherosclerosis (159), and there already exists vested interest in translating these findings into therapies to boost PC-specific Abs in order to reduce cardiovascular disease risk (160, 161). The therapeutic benefits of natural IgM may be harnessed through passive immunotherapy with monoclonal natural IgM antibodies, or through infusion of pooled polyclonal serum IgM preparations such as those already in use for intravenous Immunoglobulin (IVIg) or cocktails of therapeutic immunoglobulin (162). Infusion of a recombinant natural IgM antibody shows great promise in promoting the remylenination of spinal neurons in mouse models of MS (163). Immunization with key polysaccharide antigens may also be used to promote the establishment of clonal specificities in the adult B cell repertoire (162).
Profiling global glycan-reactive antibody specificities in autoimmune disease settings has already yielded significant correlations of disease status with PS-specific antibody responses (61–65). Further profiling of individuals affected by autoimmune diseases, as well as healthy individuals, via glycan microarray techniques will contribute to our developing understanding of healthy natural antibody repertoires, and aid in identifying those patients who may be predisposed to various autoimmune conditions. Identification of such underlying defects in natural antibody repertoire enables development of potential therapies aimed to drive specific clonal expansion of B cells that are protective in autoimmune and allergic diseases. Most notable is growing evidence that nAb clonotypes are restricted in the human population (4, 119). In the future, V(D)J-gene utilization signatures in human memory B cell compartments may be associated with susceptibility to aberrant immunological phenomena. Furthermore, development of anti-idiotypic reagents, restricted for highly specific clonal features, will yield highly sensitive detection of peripheral blood B cell clonotypes and circulating antibodies. Natural antibodies of the IgM isotype play important roles in health and disease that we are only beginning to understand, and they represent a novel platform with great potential to predict and prevent aberrant immunological processes.
Acknowledgments
We would like to thank Denise Kaminski for her thorough review of the manuscript. This work was supported by research funds from the National Institutes of Health (NIH) Grant AI14782-37 and AI100005-03, and funds from the Juvenile Diabetes Research Foundation and the American Asthma Foundation to JFK. J.S.N. was supported by F31AI120500 and T32A1007051.
Footnotes
The authors possess no potential conflicts of interest at the time of manuscript preparation.
References
- 1.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 2.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 3.Kin NW, Stefanov EK, Dizon BL, Kearney JF. Antibodies generated against conserved antigens expressed by bacteria and allergen-bearing fungi suppress airway disease. J Immunol. 2012;189:2246–2256. doi: 10.4049/jimmunol.1200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gronwall C, Silverman GJ. Natural IgM: beneficial autoantibodies for the control of inflammatory and autoimmune disease. J Clin Immunol. 2014;34(Suppl 1):S12–S21. doi: 10.1007/s10875-014-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney JF, Patel P, Stefanov EK, King RG. Natural antibody repertoires: development and functional role in inhibiting allergic airway disease. Annu Rev Immunol. 2015;33:475–504. doi: 10.1146/annurev-immunol-032713-120140. [DOI] [PubMed] [Google Scholar]
- 6.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 8.Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:5–15. doi: 10.1007/s12016-011-8285-8. [DOI] [PubMed] [Google Scholar]
- 9.Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y. Infections and autoimmunity--friends or foes? Trends Immunol. 2009;30:409–414. doi: 10.1016/j.it.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein MF, Goldstein AL, Dunsky EH, Dvorin DJ, Belecanech GA, Shamir K. Selective IgM immunodeficiency: retrospective analysis of 36 adult patients with review of the literature. Ann Allergy Asthma Immunol. 2006;97:717–730. doi: 10.1016/S1081-1206(10)60962-3. [DOI] [PubMed] [Google Scholar]
- 12.Louis AG, Gupta S. Primary selective IgM deficiency: an ignored immunodeficiency. Clin Rev Allergy Immunol. 2014;46:104–111. doi: 10.1007/s12016-013-8375-x. [DOI] [PubMed] [Google Scholar]
- 13.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briles DE, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochsenbein AF, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 16.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol. 2010;184:5755–5767. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cywes-Bentley C, et al. Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proc Natl Acad Sci U S A. 2013;110:E2209–E2218. doi: 10.1073/pnas.1303573110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickman G, Julian L, Olson MF. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012;19:735–742. doi: 10.1038/cdd.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. J Exp Med. 2002;196:655–665. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. The Journal of biological chemistry. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 24.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 25.Chou MY, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrenstein MR, Cook HT, Neuberger MS. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J Exp Med. 2000;191:1253–1258. doi: 10.1084/jem.191.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binder CJ, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 28.Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, et al. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel PS, Kearney JF. Neonatal Exposure to Pneumococcal Phosphorylcholine Modulates the Development of House Dust Mite Allergy during Adult Life. J Immunol. 2015;194:5838–5850. doi: 10.4049/jimmunol.1500251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeannin P, Jaillon S, Delneste Y. Pattern recognition receptors in the immune response against dying cells. Curr Opin Immunol. 2008;20:530–537. doi: 10.1016/j.coi.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Carroll MC, Holers VM. Innate autoimmunity. Adv Immunol. 2005;86:137–157. doi: 10.1016/S0065-2776(04)86004-8. [DOI] [PubMed] [Google Scholar]
- 34.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert ML, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843–854. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gronwall C, et al. MAPK phosphatase-1 is required for regulatory natural autoantibody-mediated inhibition of TLR responses. Proc Natl Acad Sci U S A. 2012;109:19745–19750. doi: 10.1073/pnas.1211868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lobo PI, et al. Natural IgM anti-leukocyte autoantibodies attenuate excess inflammation mediated by innate and adaptive immune mechanisms involving Th-17. J Immunol. 2012;188:1675–1685. doi: 10.4049/jimmunol.1101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai H, Zhang Y, Lv P, Gao XM. A study on the glycan specificity of natural antibody repertoires in rodents. Cell Mol Immunol. 2009;6:453–459. doi: 10.1038/cmi.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huflejt ME, et al. Anti-carbohydrate antibodies of normal sera: findings, surprises and challenges. Mol Immunol. 2009;46:3037–3049. doi: 10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Bovin N, et al. Repertoire of human natural anti-glycan immunoglobulins. Do we have auto-antibodies? Biochim Biophys Acta. 2012;1820:1373–1382. doi: 10.1016/j.bbagen.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Spalter SH, Kaveri SV, Bonnin E, Mani JC, Cartron JP, Kazatchkine MD. Normal human serum contains natural antibodies reactive with autologous ABO blood group antigens. Blood. 1999;93:4418–4424. [PubMed] [Google Scholar]
- 44.Schneider C, et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci Transl Med. 2015;7:269ra261. doi: 10.1126/scitranslmed.3010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroeder HW, Jr, Hillson JL, Perlmutter RM. Early restriction of the human antibody repertoire. Science. 1987;238:791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder HW, Jr, Wang JY. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990;87:6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emmrich F, Greger B, Eichmann K. A cross-reacting human idiotype (B17) associated with antibodies to N-acetyl-D-glucosamine. Specificity, immunoglobulin class association, and distribution in the population. Eur J Immunol. 1983;13:273–278. doi: 10.1002/eji.1830130402. [DOI] [PubMed] [Google Scholar]
- 48.Rabinovich GA, van Kooyk Y, Cobb BA. Glycobiology of immune responses. Ann N Y Acad Sci. 2012;1253:1–15. doi: 10.1111/j.1749-6632.2012.06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emmrich F, Schilling B, Eichmann K. Human immune response to group A streptococcal carbohydrate (A-CHO). I. Quantitative and qualitative analysis of the A-CHO-specific B cell population responding in vitro to polyclonal and specific activation. J Exp Med. 1985;161:547–562. doi: 10.1084/jem.161.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang WH, Aziz PV, Heithoff DM, Mahan MJ, Smith JW, Marth JD. An intrinsic mechanism of secreted protein aging and turnover. Proc Natl Acad Sci U S A. 2015;112:13657–13662. doi: 10.1073/pnas.1515464112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meesmann HM, et al. Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J Cell Sci. 2010;123:3347–3356. doi: 10.1242/jcs.066696. [DOI] [PubMed] [Google Scholar]
- 52.Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- 53.Yamanaka M, Eda S, Beppu M. Carbohydrate chains and phosphatidylserine successively work as signals for apoptotic cell removal. Biochem Biophys Res Commun. 2005;328:273–280. doi: 10.1016/j.bbrc.2004.12.171. [DOI] [PubMed] [Google Scholar]
- 54.Franz S, et al. Lectins detect changes of the glycosylation status of plasma membrane constituents during late apoptosis. Cytometry A. 2006;69:230–239. doi: 10.1002/cyto.a.20206. [DOI] [PubMed] [Google Scholar]
- 55.Russell L, Waring P, Beaver JP. Increased cell surface exposure of fucose residues is a late event in apoptosis. Biochem Biophys Res Commun. 1998;250:449–453. doi: 10.1006/bbrc.1998.9337. [DOI] [PubMed] [Google Scholar]
- 56.Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002;9:493–504. doi: 10.1038/sj.cdd.4400987. [DOI] [PubMed] [Google Scholar]
- 57.Ise H, Goto M, Komura K, Akaike T. Engulfment and clearance of apoptotic cells based on a GlcNAc-binding lectin-like property of surface vimentin. Glycobiology. 2012;22:788–805. doi: 10.1093/glycob/cws052. [DOI] [PubMed] [Google Scholar]
- 58.Cervera A, et al. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS One. 2010;5:e8433. doi: 10.1371/journal.pone.0008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 60.Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985;314:53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- 61.Engelbertsen D, et al. Low Levels of IgM Antibodies against an Advanced Glycation Endproduct-Modified Apolipoprotein B100 Peptide Predict Cardiovascular Events in Nondiabetic Subjects. J Immunol. 2015;195:3020–3025. doi: 10.4049/jimmunol.1402869. [DOI] [PubMed] [Google Scholar]
- 62.Wang D, et al. Uncovering cryptic glycan markers in multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) Drug Dev Res. 2014;75:172–188. doi: 10.1002/ddr.21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freedman MS, Laks J, Dotan N, Altstock RT, Dukler A, Sindic CJ. Anti-alpha-glucose-based glycan IgM antibodies predict relapse activity in multiple sclerosis after the first neurological event. Mult Scler. 2009;15:422–430. doi: 10.1177/1352458508101944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dotan N, Altstock RT, Schwarz M, Dukler A. Anti-glycan antibodies as biomarkers for diagnosis and prognosis. Lupus. 2006;15:442–450. doi: 10.1191/0961203306lu2331oa. [DOI] [PubMed] [Google Scholar]
- 65.Dotan I, et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn's disease. Gastroenterology. 2006;131:366–378. doi: 10.1053/j.gastro.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 66.Herzenberg LA, et al. The Ly-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 67.Kearney JF. Innate-like B cells. Springer Semin Immunopathol. 2005;26:377–383. doi: 10.1007/s00281-004-0184-0. [DOI] [PubMed] [Google Scholar]
- 68.Won WJ, Bachmann MF, Kearney JF. CD36 is differentially expressed on B cell subsets during development and in responses to antigen. J Immunol. 2008;180:230–237. doi: 10.4049/jimmunol.180.1.230. [DOI] [PubMed] [Google Scholar]
- 69.Herzenberg LA, Tung JW. B cell lineages: documented at last! Nat Immunol. 2006;7:225–226. doi: 10.1038/ni0306-225. [DOI] [PubMed] [Google Scholar]
- 70.Fairfax KA, et al. Different kinetics of blimp-1 induction in B cell subsets revealed by reporter gene. J Immunol. 2007;178:4104–4111. doi: 10.4049/jimmunol.178.7.4104. [DOI] [PubMed] [Google Scholar]
- 71.Maity PC, Blount A, Jumaa H, Ronneberger O, Lillemeier BF, Reth M. B cell antigen receptors of the IgM and IgD classes are clustered in different protein islands that are altered during B cell activation. Sci Signal. 2015;8:ra93. doi: 10.1126/scisignal.2005887. [DOI] [PubMed] [Google Scholar]
- 72.Kaminski DA, Stavnezer J. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. J Immunol. 2006;177:6025–6029. doi: 10.4049/jimmunol.177.9.6025. [DOI] [PubMed] [Google Scholar]
- 73.Savage HP, Baumgarth N. Characteristics of natural antibody-secreting cells. Ann N Y Acad Sci. 2015 doi: 10.1111/nyas.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin F, Chen X, Shu F, Kearney JF. Development of the mouse B-cell repertoire. Ann N Y Acad Sci. 1995;764:207–221. doi: 10.1111/j.1749-6632.1995.tb55829.x. [DOI] [PubMed] [Google Scholar]
- 75.Benedict CL, Gilfillan S, Thai TH, Kearney JF. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev. 2000;175:150–157. [PubMed] [Google Scholar]
- 76.Hardy RR, Wei CJ, Hayakawa K. Selection during development of VH11+ B cells: a model for natural autoantibody-producing CD5+ B cells. Immunol Rev. 2004;197:60–74. doi: 10.1111/j.0105-2896.2004.0100.x. [DOI] [PubMed] [Google Scholar]
- 77.Wasserman R, et al. A novel mechanism for B cell repertoire maturation based on response by B cell precursors to pre-B receptor assembly. J Exp Med. 1998;187:259–264. doi: 10.1084/jem.187.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marshall AJ, Doyen N, Bentolila LA, Paige CJ, Wu GE. Terminal deoxynucleotidyl transferase expression during neonatal life alters D(H) reading frame usage and Ig-receptor-dependent selection of V regions. J Immunol. 1998;161:6657–6663. [PubMed] [Google Scholar]
- 79.Martin F, Chen X, Kearney JF. Development of VH81X transgene-bearing B cells in fetus and adult: sites for expansion and deletion in conventional and CD5/B1 cells. Int Immunol. 1997;9:493–505. doi: 10.1093/intimm/9.4.493. [DOI] [PubMed] [Google Scholar]
- 80.Freitas AA, Viale AC, Sundblad A, Heusser C, Coutinho A. Normal serum immunoglobulins participate in the selection of peripheral B-cell repertoires. Proc Natl Acad Sci U S A. 1991;88:5640–5644. doi: 10.1073/pnas.88.13.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 82.Hayakawa K, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 83.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 84.Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- 85.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci U S A. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimoto M, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Y, et al. Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J Exp Med. 2015;212:569–580. doi: 10.1084/jem.20141510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ding C, et al. Siglecg limits the size of B1a B cell lineage by down-regulating NFkappaB activation. PLoS One. 2007;2:e997. doi: 10.1371/journal.pone.0000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rajewsky K. The Herzenberg lecture: how to make a B-1 cell? Ann N Y Acad Sci. 2015 doi: 10.1111/nyas.12767. [DOI] [PubMed] [Google Scholar]
- 92.Inaoki M, Sato S, Weintraub BC, Goodnow CC, Tedder TF. CD19-regulated signaling thresholds control peripheral tolerance and autoantibody production in B lymphocytes. J Exp Med. 1997;186:1923–1931. doi: 10.1084/jem.186.11.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pao LI, et al. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 94.Ferry H, Crockford TL, Leung JC, Cornall RJ. Signals from a self-antigen induce positive selection in early B cell ontogeny but are tolerogenic in adults. J Immunol. 2006;176:7402–7411. doi: 10.4049/jimmunol.176.12.7402. [DOI] [PubMed] [Google Scholar]
- 95.Hoffmann A, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 96.Stall AM, Adams S, Herzenberg LA, Kantor AB. Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann N Y Acad Sci. 1992;651:33–43. doi: 10.1111/j.1749-6632.1992.tb24591.x. [DOI] [PubMed] [Google Scholar]
- 97.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 98.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol. 2009;183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Y, et al. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Sci U S A. 2012;109:5388–5393. doi: 10.1073/pnas.1121627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang Y, et al. Antigen-specific antibody responses in B-1a and their relationship to natural immunity. Proc Natl Acad Sci U S A. 2012;109:5382–5387. doi: 10.1073/pnas.1121631109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Foote JB, Mahmoud TI, Vale AM, Kearney JF. Long-term maintenance of polysaccharide-specific antibodies by IgM-secreting cells. J Immunol. 2012;188:57–67. doi: 10.4049/jimmunol.1100783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baumgarth N, Waffarn EE, Nguyen TT. Natural and induced B-1 cell immunity to infections raises questions of nature versus nurture. Ann N Y Acad Sci. 2015 doi: 10.1111/nyas.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bortnick A, Allman D. What is and what should always have been: long-lived plasma cells induced by T cell-independent antigens. J Immunol. 2013;190:5913–5918. doi: 10.4049/jimmunol.1300161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol. 2003;171:5406–5414. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- 105.Reynolds AE, Kuraoka M, Kelsoe G. Natural IgM is produced by CD5- plasma cells that occupy a distinct survival niche in bone marrow. J Immunol. 2015;194:231–242. doi: 10.4049/jimmunol.1401203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi W, et al. Transcriptional profiling of mouse B cell terminal differentiation defines a signature for antibody-secreting plasma cells. Nat Immunol. 2015;16:663–673. doi: 10.1038/ni.3154. [DOI] [PubMed] [Google Scholar]
- 107.Hamilton AM, Kearney JF. Effects of IgM allotype suppression on serum IgM levels, B-1 and B-2 cells, and antibody responses in allotype heterozygous F1 mice. Dev Immunol. 1994;4:27–41. doi: 10.1155/1994/45728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mosier DE. Induction of B cell priming by neonatal injection of mice with thymic-independent (type 2) antigens. J Immunol. 1978;121:1453–1459. [PubMed] [Google Scholar]
- 109.Kearney JF, McCarthy MT, Stohrer R, Benjamin WH, Jr, Briles DE. Induction of germ-line anti-alpha 1–3 dextran antibody responses in mice by members of the Enterobacteriaceae family. J Immunol. 1985;135:3468–3472. [PubMed] [Google Scholar]
- 110.Mahmoud TI, Schroeder HW, Jr, Kearney JF. Limiting CDR-H3 diversity abrogates the antibody response to the bacterial polysaccharide alpha 1-->3 dextran. J Immunol. 2011;187:879–886. doi: 10.4049/jimmunol.1100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mahmoud TI, Kearney JF. Terminal deoxynucleotidyl transferase is required for an optimal response to the polysaccharide alpha-1,3 dextran. J Immunol. 2010;184:851–858. doi: 10.4049/jimmunol.0902791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stohrer R, Lee MC, Kearney JF. Analysis of the anti-alpha 1 leads to 3 dextran response with monoclonal anti-idiotype antibodies. J Immunol. 1983;131:1375–1379. [PubMed] [Google Scholar]
- 113.Kudlow JE. Post-translational modification by O-GlcNAc: another way to change protein function. Journal of cellular biochemistry. 2006;98:1062–1075. doi: 10.1002/jcb.20926. [DOI] [PubMed] [Google Scholar]
- 114.Lutz CT, et al. Molecular dissection of the murine antibody response to streptococcal group A carbohydrate. J Exp Med. 1987;165:531–545. doi: 10.1084/jem.165.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greenspan NS, Davie JM. Serologic and topographic characterization of idiotopes on murine monoclonal anti-streptococcal group A carbohydrate antibodies. J Immunol. 1985;134:1065–1072. [PubMed] [Google Scholar]
- 116.Zenke G, Eichmann K, Emmrich F. Characterization of a major human antibody clonotype (1A) by monoclonal antibodies to combining site-associated idiotopes. Eur J Immunol. 1984;14:164–170. doi: 10.1002/eji.1830140211. [DOI] [PubMed] [Google Scholar]
- 117.Emmrich F, Zenke G, Eichmann K. Isotype restriction of idiotopes associated with human anti-streptococcal A carbohydrate antibodies. Eur J Immunol. 1986;16:542–546. doi: 10.1002/eji.1830160514. [DOI] [PubMed] [Google Scholar]
- 118.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev. 2014;258:241–258. doi: 10.1111/imr.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang L, Radic MZ, Galili U. Human anti-Gal heavy chain genes. Preferential use of VH3 and the presence of somatic mutations. J Immunol. 1995;155:1276–1285. [PubMed] [Google Scholar]
- 120.Ohdan H, et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Gal alpha 1,3Gal epitopes in alpha 1,3-galactosyltransferase-deficient mice. J Immunol. 2000;165:5518–5529. doi: 10.4049/jimmunol.165.10.5518. [DOI] [PubMed] [Google Scholar]
- 121.Benatuil L, et al. Ig knock-in mice producing anti-carbohydrate antibodies: breakthrough of B cells producing low affinity anti-self antibodies. J Immunol. 2008;180:3839–3848. doi: 10.4049/jimmunol.180.6.3839. [DOI] [PubMed] [Google Scholar]
- 122.Parker W, Yu PB, Holzknecht ZE, Lundberg K, Buckley RH, Platt JL. Specificity and function of "natural" antibodies in immunodeficient subjects: clues to B cell lineage and development. J Clin Immunol. 1997;17:311–321. doi: 10.1023/a:1027378716015. [DOI] [PubMed] [Google Scholar]
- 123.Yilmaz B, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014;159:1277–1289. doi: 10.1016/j.cell.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Casali P, Schettino EW. Structure and function of natural antibodies. Curr Top Microbiol Immunol. 1996;210:167–179. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- 125.Sigal NH, Gearhart PJ, Klinman NR. The frequency of phosphorylcholine-specific B cells in conventional and germfree BALB/C mice. J Immunol. 1975;114:1354–1358. [PubMed] [Google Scholar]
- 126.Corte-Real J, et al. Irf4 is a positional and functional candidate gene for the control of serum IgM levels in the mouse. Genes Immun. 2009;10:93–99. doi: 10.1038/gene.2008.73. [DOI] [PubMed] [Google Scholar]
- 127.Bos NA, Meeuwsen CG, Wostmann BS, Pleasants JR, Benner R. The influence of exogenous antigenic stimulation on the specificity repertoire of background immunoglobulin-secreting cells of different isotypes. Cell Immunol. 1988;112:371–380. doi: 10.1016/0008-8749(88)90306-1. [DOI] [PubMed] [Google Scholar]
- 128.Hashimoto K, Handa H, Umehara K, Sasaki S. Germfree mice reared on an "antigen-free" diet. Lab Anim Sci. 1978;28:38–45. [PubMed] [Google Scholar]
- 129.Springer GF, Horton RE. Blood group isoantibody stimulation in man by feeding blood group-active bacteria. J Clin Invest. 1969;48:1280–1291. doi: 10.1172/JCI106094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol. 1997;27:1557–1563. doi: 10.1002/eji.1830270635. [DOI] [PubMed] [Google Scholar]
- 131.Bos NA, Meeuwsen CG, Hooijkaas H, Benner R, Wostmann BS, Pleasants JR. Early development of Ig-secreting cells in young of germ-free BALB/c mice fed a chemically defined ultrafiltered diet. Cell Immunol. 1987;105:235–245. doi: 10.1016/0008-8749(87)90071-2. [DOI] [PubMed] [Google Scholar]
- 132.Bos NA, et al. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989;19:2335–2339. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- 133.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Comstock LE. Importance of glycans to the host-bacteroides mutualism in the mammalian intestine. Cell Host Microbe. 2009;5:522–526. doi: 10.1016/j.chom.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 135.Goto Y, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345:1254009. doi: 10.1126/science.1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 137.McCoy KD, et al. Natural IgE production in the absence of MHC Class II cognate help. Immunity. 2006;24:329–339. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 138.Kroese FG, Butcher EC, Stall AM, Herzenberg LA. A major peritoneal reservoir of precursors for intestinal IgA plasma cells. Immunol Invest. 1989;18:47–58. doi: 10.3109/08820138909112226. [DOI] [PubMed] [Google Scholar]
- 139.Kroese FG, Butcher EC, Stall AM, Lalor PA, Adams S, Herzenberg LA. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1:75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- 140.Thurnheer MC, Zuercher AW, Cebra JJ, Bos NA. B1 cells contribute to serum IgM, but not to intestinal IgA, production in gnotobiotic Ig allotype chimeric mice. J Immunol. 2003;170:4564–4571. doi: 10.4049/jimmunol.170.9.4564. [DOI] [PubMed] [Google Scholar]
- 141.Bunker JJ, et al. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity. 2015;43:541–553. doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fransen F, et al. BALB/c and C57BL/6 Mice Differ in Polyreactive IgA Abundance, which Impacts the Generation of Antigen-Specific IgA and Microbiota Diversity. Immunity. 2015;43:527–540. doi: 10.1016/j.immuni.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 143.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 144.Diana J, et al. Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J Immunol. 2013;191:2335–2343. doi: 10.4049/jimmunol.1300864. [DOI] [PubMed] [Google Scholar]
- 145.Bollinger RR, Everett ML, Wahl SD, Lee YH, Orndorff PE, Parker W. Secretory IgA and mucin-mediated biofilm formation by environmental strains of Escherichia coli: role of type 1 pili. Mol Immunol. 2006;43:378–387. doi: 10.1016/j.molimm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 146.D'Auria G, et al. Active and secreted IgA-coated bacterial fractions from the human gut reveal an under-represented microbiota core. Sci Rep. 2013;3:3515. doi: 10.1038/srep03515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Magri G, et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014;15:354–364. doi: 10.1038/ni.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bao S, et al. Intestinal IgA plasma cells of the B1 lineage are IL-5 dependent. Immunology. 1998;94:181–188. doi: 10.1046/j.1365-2567.1998.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.El Kaissouni J, Bene MC, Thionnois S, Monin P, Vidailhet M, Faure GC. Maturation of B cells in the lamina propria of human gut and bronchi in the first months of human life. Dev Immunol. 1998;5:153–159. doi: 10.1155/1998/42138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Golby S, Hackett M, Boursier L, Dunn-Walters D, Thiagamoorthy S, Spencer J. B cell development and proliferation of mature B cells in human fetal intestine. J Leukoc Biol. 2002;72:279–284. [PubMed] [Google Scholar]
- 152.Rogosch T, et al. IgA response in preterm neonates shows little evidence of antigen-driven selection. J Immunol. 2012;189:5449–5456. doi: 10.4049/jimmunol.1103347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lindner C, et al. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J Exp Med. 2012;209:365–377. doi: 10.1084/jem.20111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lindner C, et al. Diversification of memory B cells drives the continuous adaptation of secretory antibodies to gut microbiota. Nat Immunol. 2015 doi: 10.1038/ni.3213. [DOI] [PubMed] [Google Scholar]
- 155.Gronwall C, Vas J, Silverman GJ. Protective Roles of Natural IgM Antibodies. Front Immunol. 2012;3:66. doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kroese FG, de Waard R, Bos NA. B-1 cells and their reactivity with the murine intestinal microflora. Semin Immunol. 1996;8:11–18. doi: 10.1006/smim.1996.0003. [DOI] [PubMed] [Google Scholar]
- 157.Wesemann DR, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501:112–115. doi: 10.1038/nature12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alhabbab R, et al. Diversity of gut microflora is required for the generation of B cell with regulatory properties in a skin graft model. Sci Rep. 2015;5:11554. doi: 10.1038/srep11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frostegard J. Low level natural antibodies against phosphorylcholine: a novel risk marker and potential mechanism in atherosclerosis and cardiovascular disease. Clin Immunol. 2010;134:47–54. doi: 10.1016/j.clim.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 160.van Leeuwen M, Damoiseaux J, Duijvestijn A, Tervaert JW. The therapeutic potential of targeting B cells and anti-oxLDL antibodies in atherosclerosis. Autoimmunity reviews. 2009;9:53–57. doi: 10.1016/j.autrev.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 161.Tsiantoulas D, Sage AP, Mallat Z, Binder CJ. Targeting B cells in atherosclerosis: closing the gap from bench to bedside. Arterioscler Thromb Vasc Biol. 2015;35:296–302. doi: 10.1161/ATVBAHA.114.303569. [DOI] [PubMed] [Google Scholar]
- 162.Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol. 2012;188:939–945. doi: 10.4049/jimmunol.1102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Warrington AE, Bieber AJ, Ciric B, Pease LR, Van Keulen V, Rodriguez M. A recombinant human IgM promotes myelin repair after a single, very low dose. J Neurosci Res. 2007;85:967–976. doi: 10.1002/jnr.21217. [DOI] [PubMed] [Google Scholar]