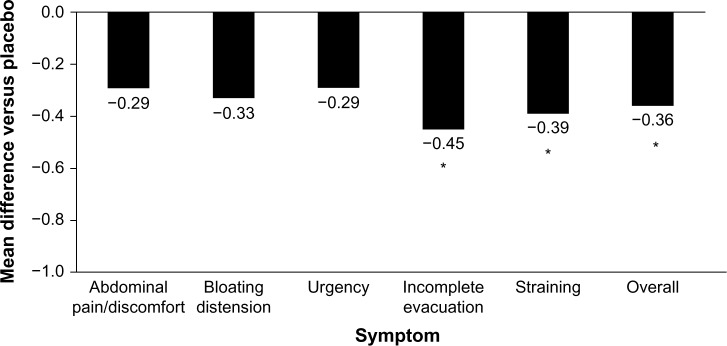
Figure 2.
Reduction of IBS symptoms with Bifidobacterium infantis capsule administered once daily vs placebo.
Notes: Females with IBS-D recruited from primary care centers were randomized to receive B. infantis 35624 1×108 (n=49) or placebo (n=56) once daily for 4 weeks. Data are least-squares mean changes from baseline at week 4. *P<0.03 vs placebo. Data from Whorwell et al.54
Abbreviations: IBS, irritable bowel syndrome; IBS-D, diarrhea-predominant IBS.