Abstract
Gliomas are among the most commonly diagnosed central nervous system tumors. Celecoxib has been utilized with success in the treatment of several types of cancer, including gliomas. The present study examined the antiproliferative effects of celecoxib and its benzimidazole-based analog, LLW-3-6, when used as co-treatments with sulfasalazine against human glioma LN18 cells. At 48-hour treatment, the glioma cells maintained 60% viability in the presence of celecoxib or LLW-3-6 at the maximum concentration tested (40 μM). Co-treatment of the glioma cells with a non-lethal dose (50 μM) of sulfasalazine and either celecoxib or LLW-3-6 (administered at different concentrations) resulted in improved inhibition of cell viability. The concentration of the molecules needed to reduce cell growth in the combined treatments was significantly less than that needed when either molecule was administered independently. Based on computational values, LLW-3-6 has physiochemical characteristics that should allow for improved bioavailability in comparison to that of celecoxib.
Keywords: Cyclooxygenase-2, sulfasalazine, celecoxib, benzimidazole, combination therapy, synergistic effects, brain cancer, glioma, LN18
Cancer that originate in the brain and other locations in the central nervous system (CNS) are among the rarest forms of the disease. In 2015, it is estimated there will be 22,850 new cases of cancer of the CNS, representing fewer than 2% of incidences of cancer predicted for all sites (1). Despite the rarity of cancer found in the CNS, the malignancies are associated with a 67% estimated mortality rate, matching that of cancer in the respiratory system, responsible for the largest number of cancer deaths annually. Gliomas are the most frequently diagnosed type of CNS cancer (2, 3). Overall, outcomes for individuals suffering with gliomas have shown insignificant improvement despite progress towards developing more effective treatments, deeming the disease incurable (4). Even with the aggressive treatment options currently available, radioresistance and tumor recurrence continue to contribute to poor prognosis for patients with glioma. In addition, treatment of the late stage disease using a single-agent chemotherapeutic is limited due to the low efficacy or low bioavailability of many promising drug candidates.
Cyclooxygenase-2 (COX2) inhibitors such as celecoxib (Figure 1) have rapidly emerged as a new-generation therapeutic for various cancer types, including gliomas. The molecule has been used as a single agent and in combination with conventional therapies to address the disease (5–7). Protocols using celecoxib have been reported to enhance radiosensitivity and reduced drug resistance in some patients (8, 9). Celecoxib was shown to reduce glioma cell viability by inducing DNA damage, leading to p53-dependent G1 cell-cycle arrest and autophagy (10). However, the use of celecoxib in patients with glioblastoma has seen mixed results, due in part to the low potential of the drug to cross the blood–brain barrier (BBB) (11, 12). In vitro pharmacokinetics studies aimed at better understanding the mechanism of action for celecoxib have found that after oral administration, it is rapidly absorbed and achieves maximum concentration in the blood in approximately 3 hours (13, 14). The plasma concentration achieved in humans is below what is typically needed for in vitro efficacy in most cells (15, 16). In addition, the molecule is extensively metabolized in the liver, which may further complicate in vivo outcomes. Despite concerns related to the pharmacokinetics, there remains intense interest in the anticancer effects of celecoxib and its derivatives (17, 18).
Figure 1.
Calculated Size and Lipophilicity of celecoxib and LLW-3-6. The values were obtained using ChemBio Office (PerkinElmer, Inc., Waltham, MA, USA). The lipophilicity was calculated as the octanol/water partition coefficient (logP). The size-to-lipophilicity ratios for the molecules are similar, with that of LLW-3-6 being more favorable than that of celecoxib.
Investigators have developed novel analogs aimed at overcoming the pharmacokinetics that limit the therapeutic utility of celecoxib. One such molecule is the benzimidazole-based analog LLW-3-6 (Figure 1) which was recently shown to exert a dose-dependent antiproliferative effect on cultured prostate and breast cancer cells (19–21). The analog has a size-to-lipophilicity ratio better than that of the parent molecule celecoxib (13), this is primarily owing to the absence of the trifluoromethyl group which has been replaced by the fused ring system. Both brain penetration and distribution are related to the lipophilicity of a molecule and are important in ensuring that an efficacious concentration of a drug is available to interact with the desired CNS target (22). Studies have found that molecules with high or moderate lipophilicity often exhibit favorable uptake which increases bioavailability and metabolism, leading to faster clearance and enhanced capacity for brain penetration (23). Therefore, the size to lipophilicity ratio of LLW-3-6 should allow the molecule to have improved penetration across BBB and bioavailability in the brain.
Sulfasalazine (SAS), is an effective clinically approved cystine-glutamate antiporter and serine-threonine-specific protein kinase activation inhibitor. The molecule has been found useful for both its antiproliferative effect in brain tumor patients and in treating tumor-associated seizures (1). The present study investigated the antiproliferative effects of celecoxib and LLW-3-6 on human glioma LN18 cells when each molecule is given independently and in combination with sulfasalazine. In addition, computational methods were used to gain insight into the potential CNS bioavailability of LLW-3-6 in comparison to celecoxib.
Materials and Methods
Chemicals
Celecoxib (CAS# 169590-42-5) and sulfasalazine (CAS# 599-79-1) were purchased through Sigma-Aldrich (St. Louis, MO, USA). LLW-3-6 was synthesized in the Department of Chemistry and Biochemistry at Spelman College using previous published methods (21).
Cell culture
The LN18 human glioma cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were grown in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100-U/ml penicillin, and 100-μg/ml streptomycin. The cells were grown at 37°C in a humidified atmosphere of 5% CO2. Cells were passaged by exposure to trypsin/EDTA and plated at densities of 5×105 cells per 25-mm culture flask assay.
Growth-inhibition assays
Prior to chemical treatment, cells growing near confluency were harvested, re-suspended in DMEM supplemented with FBS and seeded into 96-well at 5×104 cells per well, for the proliferation assay. Cells were incubated overnight to allow for adhesion and acclimation. Prior to treatment, cells were incubated in serum-free DMEM for at least 1 hour. The medium was subsequently removed and cells were treated with increasing concentration of celecoxib, LLW-3-6, or sulfasalazine suspended in serum-free DMEM. The cells were then incubated for 24 or 48 hours in a humidified incubator at 37°C with 5% CO2. Cell survival was determined using a modified microculture tetrazolium (MTT) assay, with the reagent WST-1 (Roche Diagnostics, Indianapolis, IN, USA). This reagent contains a tetrazolium salt, which is cleaved by metabolic enzymes to a water-soluble formazan. The cleavage is accompanied by change in color from light red to dark red. This directly correlates with the number of metabolically active cells in culture, allowing for the quantitative colorimetric assessment of cellular viability and proliferation following treatment. After the incubation period, absorbance in each well with treated cells was measured using a microplate reader at 450-nm. The absorbance of the formazan product at this wavelength, after correcting for background absorbance of control wells, was used as an index of cell proliferation. The percentage of viable cells was computed as follows:
Statistical analysis
All experiments were carried out in triplicate. Proliferation data are shown as the mean ± standard error of mean (SEM) computed from raw data) calculated using Microsoft Excel (Redmond, WA, USA).
Inhibitory effect analysis of co-therapy
Drug combinations contained a non-lethal dose of sulfasalazine (50 μM) and either celecoxib or LLW-3-6, at concentrations from 10–40-μM. Co-treatment outcomes were analyzed using the CompuSyn Software (ComboSyn, Paramus, NJ, USA) which calculates the combination index (CI), the normalized isobologram, and the correlation of the effect (Fa) to the dose reduction index (DRI) (24). In the calculation of the combination index, D represents the concentration of each drug in the independent treatment dose that produces a specific effect and Dx represents the concentration of the drug in the combination treatment dose that produces the same effect:
In the isobologram, the normalized dose of each drug is calculated as D/Dx. DRI was calculated as the inverse of the normalized dose. The log of the DRI was used in the analysis for scaling purposes.
Computational analysis of the potential for celecoxib and LLW-3-6 to cross the BBB
Physicochemical parameters associated with the bioavailability of each molecule in the brain were calculated using ACD/I-lab internet toolbox (Advanced Chemistry Development, Inc., Toronto, ON, Canada). The fraction of the molecule available for transport into the brain was measured as fu, plasma. The BBB permeability-surface area (PS) product is a prediction of the molecule’s ability to enter the brain (accounting for both active and passive transport across the BBB) and was calculated as logPS. The bioavailability of each molecule in the brain was calculated as the brain/plasma equilibration rate, log(PS×fu, brain).
Results
Viability of LN18 glioma brain cancer cells after single treatment with celecoxib and LLW-3-6
The effects of celecoxib and LLW-3-6 as single agents against LN18 glioma brain cancer cells were evaluated after 24 and 48 hours of treatment (Figure 2). Cell viability was determined based on a modified MTT assay. The molecules demonstrated minimal activity after 24 hour treatment at all concentrations (5, 10, 20, 40 μM) tested. LLW-3-6 demonstrated the most significant effect for the 48-hour assay, reducing cell viability by more than 30% at 10-μM in comparison to the 10% reduction achieved for cells treated with celecoxib at the same concentration. Likewise, LLW-3-6 inhibited cell growth approximately 40% at concentrations of 20-μM, while 40-μM of celecoxib was required to cause the same effect. However, the activity of LLW-3-6 appears to plateau at 40% growth inhibition.
Figure 2.

Effects of single-dose treatment with either celecoxib (CBX, A) or LLW-3-6 (LLW, B) and combination treatment with celecoxib/sulfasalazine (CBX-SAS) or LLW-3-6/sulfasalazine (LLW-SAS) on glioma cell survival at 24 and 48 hours. A non-lethal dose of sulfasalazine (50 μM) was used with different concentrations of celecoxib and LLW-3-6 for the co-treatments. Effects were measured following 24- and 48-hour incubation periods using a modified MTT assay.
Viability of LN18 glioma brain cancer cells after combined treatment with sulfasalazine
Viability was evaluated for LN18 glioma brain cancer cells treated with a non-lethal concentration of sulfasalazine (50 μM) in combination with celecoxib or LLW-3-6 at non-constant concentrations (5, 10, 20 and 40 μM), Figure 2. Both the celecoxib/sulfasalazine and the LLW-3-6/sulfasalazine combinations had a more significant impact on cell growth than did independent treatment with either celecoxib or LLW-3-6. After 24 hours, the celecoxib/sulfasalazine combination had no effect on the glioma cells. LLW-3-6/sulfasalazine reduced cell viability by approximately 60% in the same time frame. However, the effect observed in cells treated for 24 hours with 40-μM of LLW-3-6 in combination with sulfasalazine appears to be diminished with respect to that of the 20-μM treatment. There was a slight improvement in the treatment outcomes at 24 and 48 hours for the LLW-3-6/sulfasalazine combination. After 48 hours, the maximum concentration of LLW-3-6 in combination with sulfasalazine resulted in only 30% cell survival. Celecoxib in combination with sulfasalazine showed drastic improvements when treatment time increased from 24 to 48 hours. At the maximum celecoxib concentration tested in combination with sulfasalazine, cell viability was reduced to 5%.
Analysis of drug interactions with sulfasalazine
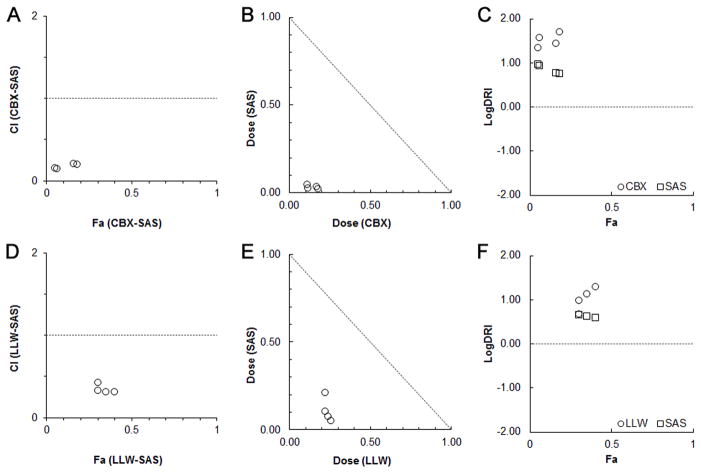
The computer-simulated CI values (Figure 3A and D) and normalized isobols (Figure 3B and E) revealed a synergistic effect when sulfasalazine was administered with celecoxib or LLW-3-6. At the maximum concentration of celecoxib (40 μM) administered with sulfasalazine, the CI value for the inhibitory effect was 0.1546. For LLW-3-6 administered with sulfasalazine, this value was 0.4321. The DRI (Figure 3C and F) indicates that there was on average a 34-fold reduction in the concentrations of celecoxib needed to produce a median effect when the celecoxib/sulfasalazine combination was used. There was a 12-fold DRI for LLW-3-6 in the co-treatment with sulfasalazine.
Figure 3.
Effects on glioma cells of treatment with celecoxib (CBX; A–C) and LLW-3-6 (LLW; D–F) in combination with sulfasalazine for a 48_hour incubation period. The combination effects were evaluated based on values generated in the CompuSyn software; combination index (CI, A and D), the normalized isobologram (B and E), and the dose reduction index (DRI, C and F). Both CI and logDRI are plotted against the effect level (Fa).
Sulfasalazine, in single-dose assays, did not display cytotoxic activity at 50-μM. However in combination with celecoxib or LLW-3-6, the activity of sulfasalazine was augmented, resulting in 7- and 4-fold increase, respectively, in efficacy for the drug.
Computational analysis of BBB transport
Physiochemical properties were calculated to predict the availability of celecoxib and LLW-3-6 in the brain (Table I). Both molecules have calculated logPS values greater than −2, indicating their potential to readily penetrate the BBB. Although the predicted rate of penetration is slightly faster for celecoxib, the plasma fu values suggest that LLW-3-6 is less likely to be bound to proteins in the blood. This could allow a higher concentration of LLW-3-6 to enter the brain in comparison to that of celecoxib. The fraction of the molecules that is bioavailable, calculated as a function of permeability rate and brain tissue binding [log(PS×fu, brain)], was found to be −2.9 and −2.7 for celecoxib and LLW-3-6, respectively. Based on this, non-specific lipid binding in the brain should be low and a peak concentration of the free molecules should be achieved rapidly.
Table I.
Physicochemical properties of celecoxib and LLW-3-6. The properties are correlated to the amount of free drug in the blood plasma (fu, plasma), the ability of the molecules to penetrate the blood–brain barrier (rate of brain penetration, logPS) and the distribution of the molecule between the brain and brain plasma (brain/plasma equilibrium rate, log(PS×fu, brain) calculated for compound in its neutral chemical state.
| Compound | fu, plasma | LogPS | Log(PS×fu, brain) |
|---|---|---|---|
| Celecoxib | 0.025 | −1.2 | −2.9 |
| LLW-3-6 | 0.013 | −1.4 | −2.7 |
Discussion
Combination therapy is considered to be a promising approach for addressing cancer. Studies have shown celecoxib to have vast potential as a chemotherapeutic agent when administered independently and in combination with other drugs (5–7). Likewise, sulfasalazine has shown notable promise as a co-treatment against the development and progression of malignancies. Of particular interest to the work reported herein, both celecoxib and sulfasalazine were shown to increase the radiosensitivity of glioblastomas, reducing cell survival (8, 9, 25). However, the combined use of celecoxib and sulfasalazine in glioma cells has not previously been reported. The present study examines the biological activity of celecoxib and LLW-3-6 in glioma cells when given with and without sulfasalazine. To address this objective, the activity of each drug was first examined independently. Little change was observed in cell viability when glioma cells were treated with either celecoxib or LLW-3-6 for 24 hours. After 48 hours, cell survival was reduced by 20% following treatment with LLW-3-6. Twice the concentration of celecoxib was needed to produce the same outcome. It should be noted that the data presented here complements the single agent cytotoxicity of celecoxib in glioma cells reported by Kang et al. (10). Overall in the absence of sulfasalazine, the ability of LLW-3-6 to inhibit the survival of glioma cells was comparable to that of celecoxib. Adding sulfasalazine to the drug treatment regimen led to increase toxicity of celecoxib in the glioma cell line. In addition, enhanced cytotoxic effects were observed for LLW-3-6 when given in combination with sulfasalazine.
The treatment outcomes seem to suggest that sulfasalazine acts as a sensitizing agent in the glioma cells. Therefore, a computational analysis was conducted showing the nature of the drug interactions to be synergistic. The enhancement was most significant for celecoxib which demonstrated a 34-fold decrease in the concentration required to reduce cell viability in comparison to the 12-fold decrease observed for LLW-3-6. Nevertheless, the outcomes suggest that lower concentrations of each drug could be used which should limit or prevent unfavorable side effects.
The chemotherapeutic potential of celecoxib is complicated in part by its limited ability to cross the BBB (11). It has been shown that celecoxib only achieves a maximum plasma concentration below what is typically needed to demonstrate efficacy in most in vitro studies. It is of interest, therefore, to identify analogs of the drug that that has a similar efficacious profile to that of celecoxib in terms of reducing the development and progression of cancer, but with improved physiochemical properties to that of the drug. The computational data presented here suggests that LLW-3-6, having bioactivity comparable or enhanced with respect to celecoxib, may overcome the limitations to bioavailability in the CNS that have been observed for celecoxib. The calculated protein binding potential of LLW-3-6 was decreased with respect to celecoxib, which suggests that the analog could have improved bioavailability over celecoxib. This along with the cytotoxic effects of the drug suggests that the therapeutic potential of the molecule against central nervous system cancers warrants further evaluation.
Acknowledgments
The work is funded in part by the National Center on Minority Health and Health Disparities Grant# 5P20MD000215-05.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies listed.
References
- 1.Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nature Reviews Neuroscience. 2014;15:455–465. doi: 10.1038/nrn3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro-oncology. 2012;14:1171–1177. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncology. 2014;16:iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. Journal of neuro-oncology. 2012;107:359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 5.Derosa L, Galli L, Orlandi P, Fioravanti A, Di Desidero T, Fontana A, Antonuzzo A, Biasco E, Farnesi A, Marconcini R, Francia G, Danesi R, Falcone A, Bocci G. Docetaxel plus oral metronomic cyclophosphamide: A phase II study with pharmacodynamic and pharmacogenetic analyses in castration-resistant prostate cancer patients. Cancer. 2014;120:3923–3931. doi: 10.1002/cncr.28953. [DOI] [PubMed] [Google Scholar]
- 6.Liu G, Yu MY, Huang X, Zhu D, Cheng SH, Ma RS, Gu GS. Synergistic effect of celecoxib in tumor necrosis factor-related apoptosis-inducing ligand treatment in osteosarcoma cells. Mol Med Rep. 2014;10:2198–2202. doi: 10.3892/mmr.2014.2409. [DOI] [PubMed] [Google Scholar]
- 7.Weihsengruber F, Jungbauer L, Neumann M, Rappersberger K. Inoperable cutaneous squamous cell cancer: successful combination therapy with cetuximab (erbitux) and celecoxib (celebrex), prospective analysis of 2 patients (pg, 28, 2014, WIEN) J Dtsch Dermatol Ges. 2014;12:28–28. [Google Scholar]
- 8.Ma H-I, Chiou S-H, Hueng D-Y, Tai L-K, Huang P-I, Kao C-L, Chen Y-W, Sytwu H-K. Celecoxib and radioresistant glioblastoma-derived CD133+ cells: improvement in radiotherapeutic effects: Laboratory investigation. Journal of neurosurgery. 2011;114:651–662. doi: 10.3171/2009.11.JNS091396. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki K, Gerelchuluun A, Hong Z, Sun L, Zenkoh J, Moritake T, Tsuboi K. Celecoxib enhances radiosensitivity of hypoxic glioblastoma cells through endoplasmic reticulum stress. Neuro-Oncology. 2013;15:1186–1199. doi: 10.1093/neuonc/not062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang KB, Zhu C, Yong SK, Gao Q, Wong MC. Enhanced sensitivity of celecoxib in human glioblastoma cells: Induction of DNA damage leading to p53-dependent G1 cell cycle arrest and autophagy. Molecular cancer. 2009;8:66. doi: 10.1186/1476-4598-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novakova I, Subileau E-A, Toegel S, Gruber D, Lachmann B, Urban E, Chesne C, Noe CR, Neuhaus W. Transport rankings of non-steroidal antiinflammatory drugs across blood-brain barrier in vitro models. PloS one. 2014;9:e86806. doi: 10.1371/journal.pone.0086806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer B, Hartz AM, Pekcec A, Toellner K, Miller DS, Potschka H. Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Molecular pharmacology. 2008;73:1444–1453. doi: 10.1124/mol.107.041210. [DOI] [PubMed] [Google Scholar]
- 13.Paulson SK, Zhang JY, Breau AP, Hribar JD, Liu NW, Jessen SM, Lawal YM, Cogburn JN, Gresk CJ, Markos CS. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug metabolism and disposition. 2000;28:514–521. [PubMed] [Google Scholar]
- 14.Gong L, Thorn CF, Bertagnolli MM, Grosser T, Altman RB, Klein TE. Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacogenetics and genomics. 2012;22:310. doi: 10.1097/FPC.0b013e32834f94cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409–415. doi: 10.1097/00000542-200502000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Patel MI, Subbaramaiah K, Du B, Chang M, Yang P, Newman RA, Cordon-Cardo C, Thaler HT, Dannenberg AJ. Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clinical Cancer Research. 2005;11:1999–2007. doi: 10.1158/1078-0432.CCR-04-1877. [DOI] [PubMed] [Google Scholar]
- 17.Sharma V, Dixit D, Ghosh S, Sen E. COX-2 regulates the proliferation of glioma stem like cells. Neurochemistry international. 2011;59:567–571. doi: 10.1016/j.neuint.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Vera M, Barcia E, Negro S, Marcianes P, García-García L, Slowing K, Fernández-Carballido A. New celecoxib multiparticulate systems to improve glioblastoma treatment. International journal of pharmaceutics. 2014;473:518–527. doi: 10.1016/j.ijpharm.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Payton-Stewart F, Tilghman SL, Williams LG, Winfield LL. Benzimidazoles diminish ERE transcriptional activity and cell growth in breast cancer cells. Biochemical and biophysical research communications. 2014;450:1358–1362. doi: 10.1016/j.bbrc.2014.06.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yerokun T, Winfield LL. LLW-3-6 and celecoxib impacts growth in prostate cancer cells and subcellular localization of COX-2. Anticancer research. 2014;34:4755–4759. [PMC free article] [PubMed] [Google Scholar]
- 21.Winfield LL, Smith DM, Halemano K, Leggett CS. A preliminary assessment of the structure-activity relationship of benzimidazole-based anti-proliferative agents. Lett Drug Des Discov. 2008;5:369–376. doi: 10.2174/157018008785777324#sthash.5mpkAcrR.dpuf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reichel A. Addressing central nervous system (CNS) penetration in drug discovery: basics and implications of the evolving new concept. Chemistry & biodiversity. 2009;6:2030–2049. doi: 10.1002/cbdv.200900103. [DOI] [PubMed] [Google Scholar]
- 23.Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood–brain barrier penetration of molecular imaging agents. Molecular Imaging & Biology. 2003;5:376–389. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Chou T-C. Drug combination studies and their synergy quantification using the Chou- Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 25.Sleire L, Skeie BS, Netland IA, Førde HE, Dodoo E, Selheim F, Leiss L, Wang J, Heggdal J, Pedersen P-H, Enger PØ. Abstract 1789: Drug repurposing: Sulfasalazine sensitizes gliomas to gamma knife surgery by blocking cystine uptake through System XC−, leading to gluthatione depletion. Cancer Res. 2015;75:1789. doi: 10.1038/onc.2015.60. [DOI] [PubMed] [Google Scholar]