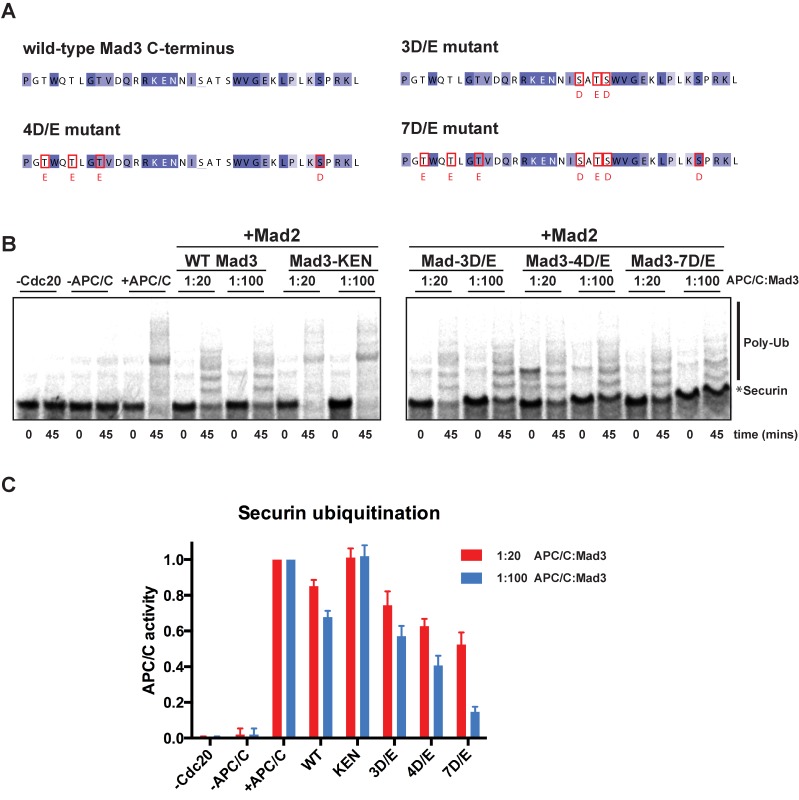
Fig 6. Mad3p modification directly affects its ability to inhibit APC/C activity.
A. Mad3p C-terminal sequence highlighting the residues mutated in the 3, 4 and 7D/E mutants. B. Fission yeast APC/C in vitro activity reactions showing the ubiquitination of securinCut2. Half of each reaction (10 μl) was taken immediately after mixing all the components and added to 4xSDS gel buffer (0 min). The remaining 10 μl were incubated at 23°C for 45 min. Control reactions, one lacking the activator Cdc20Slp1 (-Cdc20), one lacking the APC/C (-APC/C) and one containing both Cdc20Slp1 and APC/C (+APC/C), are shown together with wild-type Mad3p (WT), the double KEN box mutant (mad3-KEN1,2-AAA) and the corresponding phospho-mimic mutants at 1:20 and 1:100 APC/C:Mad3p molar ratios respectively. Purified cMad2p was added at 1:12.5 APC/C:Mad2p molar ratio in all reactions. A phosphoimager and Imagequant software were used to quantify the amount of radioactivity left in the un-modified securin band (*). C. Quantification of APC/C inhibition by wild-type (WT) Mad3p and mutants. Results from three independent experiments were combined and plotted (as mean with SEM). Data were normalised against APC/C activity containing no Mad2p or Mad3p (+APC/C).