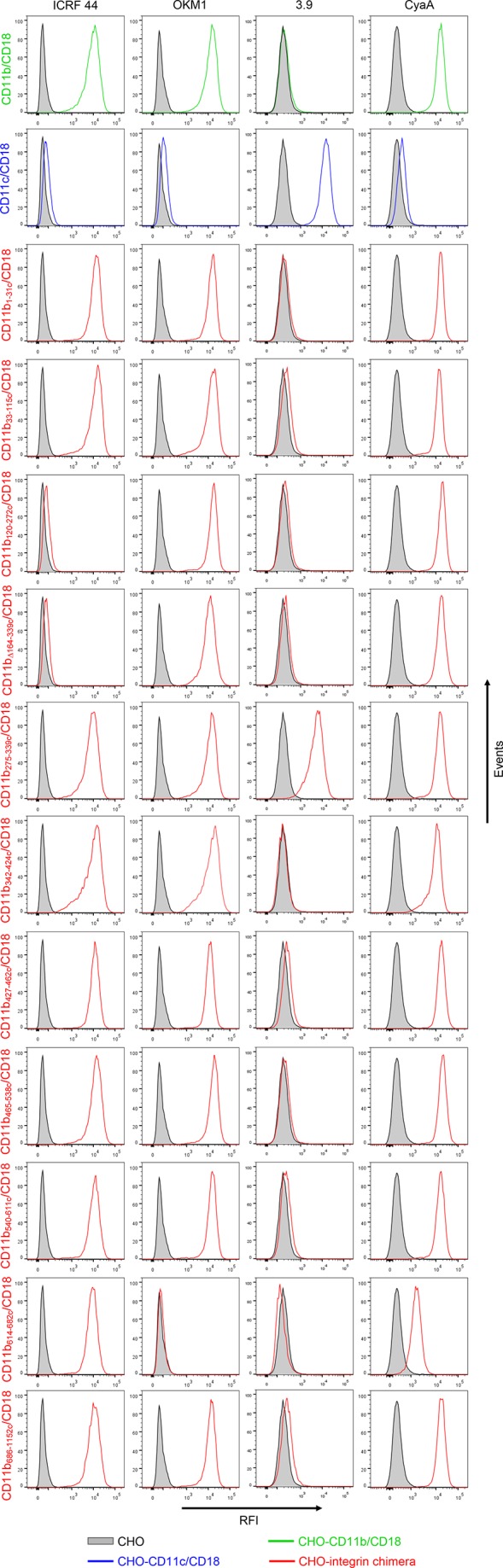
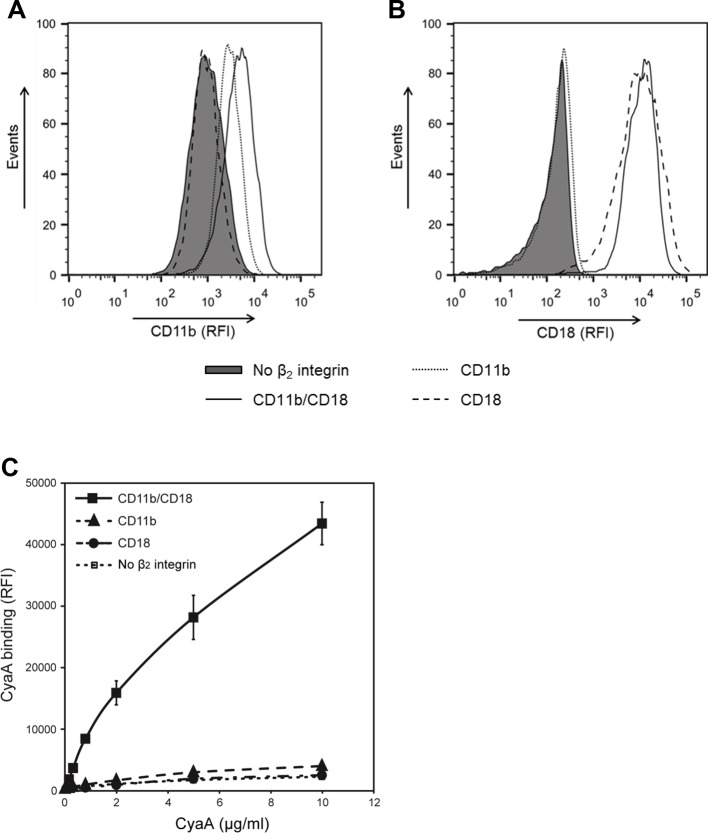
Figure 1. Residues 614-682 of CD11b are crucial for CyaA binding and penetration into cells.
(A) The CD11 subunits of β2 integrins consist of a long N-terminal extracellular domain, a single-pass transmembrane segment (TS) and a short C-terminal cytoplasmic tail, respectively. The N-terminal part of the extracellular domain harbors seven β-sheet repeats (numbers in boxes), forming a β-propeller domain, which is followed by the thigh, calf-1 and calf-2 domains. The I-domain segment, inserted between repeats 2 and 3 of the β-propeller domain, plays a critical role in interaction of the I-domain-containing integrins with their endogenous ligands. To map the CyaA binding site on the CD11b subunit, segments of CD11b (green) were systematically replaced with their CD11c counterparts (blue). In the CD11bΔ164-339 molecule, the entire I domain of CD11b was deleted. (B) 2x105 CHO cells expressing integrin molecules were incubated with 2 µg/ml of CyaA-biotin, the surface-bound toxin was labeled with streptavidin-PE and the cells were analyzed by flow cytometry. CyaA binding was expressed as percentage of toxin binding to CHO cells expressing the native form of CD11b/CD18. Each bar represents the mean value with SD of at least five independent experiments performed in duplicate or triplicate. Significantly reduced binding of CyaA to mutant integrins in comparison with intact CD11b/CD18 is indicated (****, p<0.0001; ANOVA). (C) 1x105 CHO cells expressing integrin molecules were incubated with various concentrations of CyaA and the amounts of accumulated cAMP were determined in cell lysates by ELISA. Each point represents the mean value ± SD of at least seven determinations from at least three independent experiments. Significant differences between mean values of cAMP intoxication of cells expressing intact CD11b/CD18 and mutant integrins are shown (****, p<0.0001; ANOVA).
Figure 1—figure supplement 1. Expression of the CD18 subunit on the surface of CHO cells.
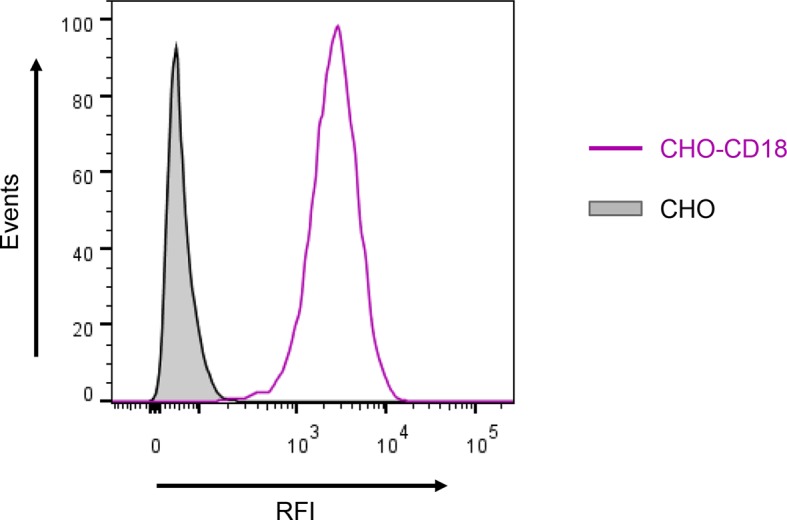
Figure 1—figure supplement 2. Binding of mAbs and CyaA to CHO cells expressing CD11b/CD18, CD11c/CD18 and the CD11b-CD11c/CD18 chimeras.