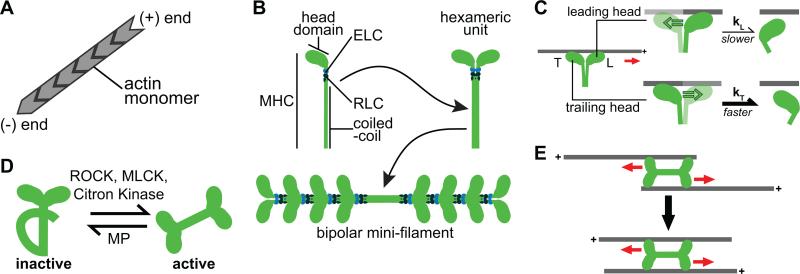
Figure 2. Properties and regulation of the actomyosin cytoskeleton.
(A) Actin monomers assemble into polar filaments with plus-ends and minus-ends. (B) Myosin is a hexameric complex composed of 2 myosin heavy chains (MHCs), 2 essential light chains (ELCs), and 2 regulatory light chains (RLCs). The MHC contains the head or motor domain, which binds and translocates F-actin, and a coiled-coil region that assembles with other coiled-coil tails to form bipolar minifilaments (bottom). (C) Leading and trailing heads experience different loads, which affect F-actin attachment lifetime. Unlike the trailing head (bottom), the leading head (top) experiences a resistive load, which slows the rate of ADP release (kL) and increases the lifetime of F-actin binding, compared to the trailing head (kT > kL). (D) Phosphorylation of the RLC by Rho-kinase (ROCK), myosin light chain kinase (MLCK), or Citron kinase promotes activation of motor activity and bipolar minifilament assembly. Dephosphorylation of the RLC by myosin phosphatase (MP) inactivates and disassembles myosin minifilaments. (E) Bipolar myosin minifilaments promote contraction of anti-parallel F-actin filaments because myosin heads walk towards the plus-ends of F-actin. In this schematic we have minimized the number of myosin heads in the bipolar minifilament for simplicity.