Figure 1.
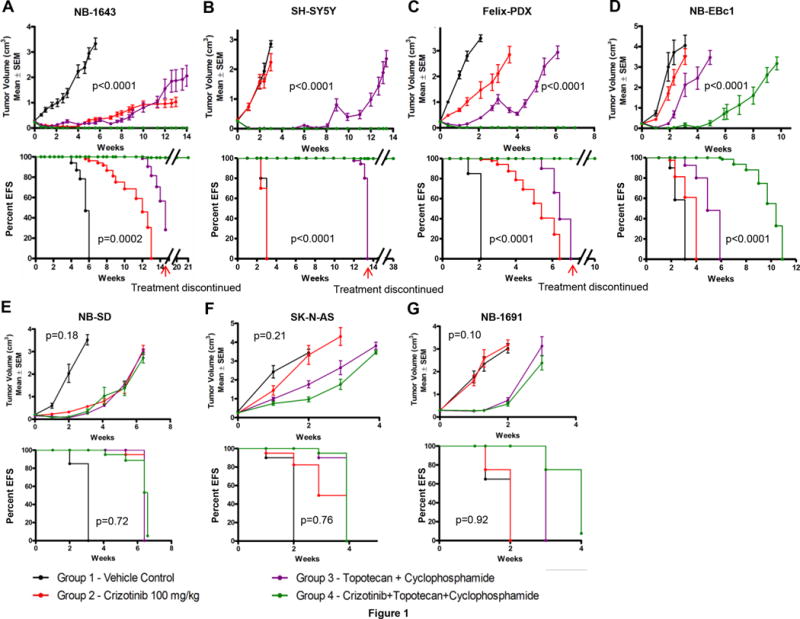
In vivo effects of crizotinib and topo/cyclo on tumor growth and event-free survival in neuroblastoma PDX and xenograft models. Female CB17 SCID mice bearing (A) NB-1643 [ALK-R1275Q, TP53-WT], (B) SH-SY5Y [ALK-F1174L, TP53-WT], (C) Felix-PDX [ALK-F1245C, TP53-WT] Patient Derived Xenograft (PDX) (D) NB-EBc1 [ALK-WT, expresses phosphorylated ALK, TP53-WT], (E) NB-SD [ALK-F1174L, TP53 mutated], (F) SK-N-AS [ALK-WT, TP53 mutated], and (G) NB-1691 [ALK-WT, TP32-WT, MDM2 amplified] human neuroblastoma xenografts were treated with vehicle, crizotinib alone (oral, 100mg/kg), topotecan (topo, 0.05 mg/kg, I.P.) plus cyclophosphamide (cyclo, 20 mg/kg, I.P.) alone, or crizotinib plus topo/cyclo. Administration of topo/cyclo was once daily for 5 days every 21 days whereas crizotinib was continuously administered on a daily basis. (Median ± S.E.M., n=10 for each data point, a mixed-effects model was used for statistical significance analysis of tumor growth delay, EFS K-M curves were compared by using a log-rank test, where p<0.05 was considered significant. The p-values reported refer to the combination treatment compared to topo/cyclo alone.)
