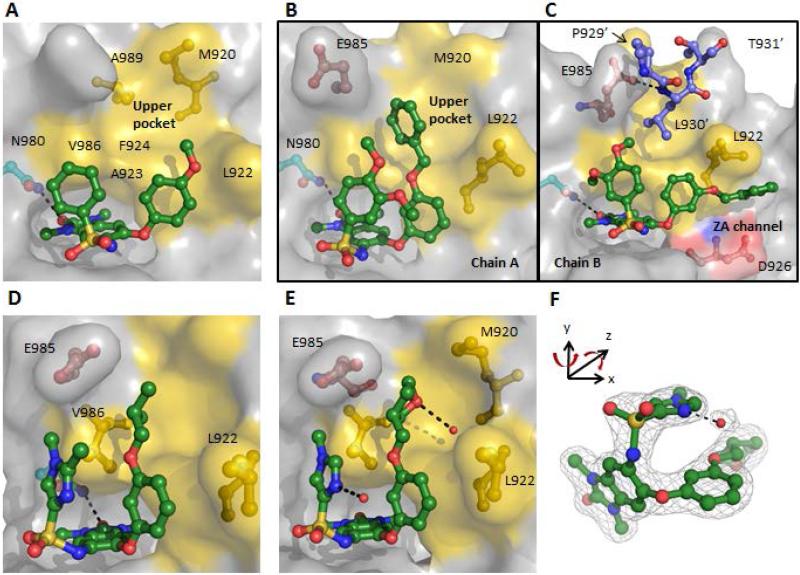
Figure 3. New unexpected binding mode enabled the design of interactions with the “upper pocket”.
(A) Cocrystal structure of 5g (2.3 Å resolution) depicting a “flipped” binding mode with the aryl-ether group now interacting with the LAF/V-shelf (PDB 4YAX). (B and C) Cocrystal structure of 7b (1.5 Å resolution) depicted in two binding conformations in the asymmetric unit (PDB 4YBM): (B) Chain A, shows the benzyl-group filling the “upper pocket”; (C) Chain B, with the benzyl-group occupying the ZA-channel and depicted in blue are three residues of Chain A TRIM24 protein with L983’ occupying the “upper pocket”. (D) Cocrystal structure of 7g (1.8 Å resolution) with the iso-butyl ether group occupying the “upper pocket” (PDB 4YBS). (E) Cocrystal stucture of 7l (1.8 Å resolution) with the 3-tetrahydropyranyl group H-bonded to a conserved water in the “upper pocket” and the imidazole H-bonded to a water that is π-stacked with the aryl-ether group (PDB 4YBT). (F) 2Fo-Fc map of 7l and the π-stacked water molecule contoured at 1.0σ (PDB 4YBT).