Fig. 1.
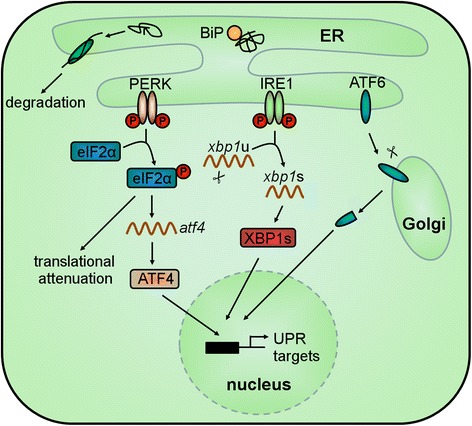
The UPR-signaling pathway. Proteins that are not properly folded within the ER are retro-translocated into the cytoplasm for degradation using the ERAD mechanism. Under ER stress conditions, unfolded proteins accumulate within the ER leading to dissociation of BiP from the ER stress sensors IRE1, PERK, and ATF6. This leads to oligomerization and autophosphorylation of IRE1 and PERK. Active IRE1 splices the xbp1 mRNA producing the spliced XBP1. Active PERK acts as a kinase of eIF2α. Under this condition, the global translation is attenuated. Thus, the protein amount entering the ER is reduced. However, the translation of atf4 mRNA is efficiently increased. Release of BiP from ATF6 permits the translocation of ATF6 to the Golgi apparatus where it is cleaved by two proteases. The resulting cytosolic portion of ATF6, ATF4, and spliced XBP1 enter the nucleus and functions as transcription factors of UPR target genes
